Patients suffering acute myocardial infarction with ST-segment elevation (STEMI) require full attention of the whole STEMI network to save their lives and to improve the quality of life after a heart attack. Close cooperation among all stakeholders on a national and regional level has to be established. The emergency medical service (EMS) and direct transportation to the 24 hours a day, seven days a week(24/7) catheterisation laboratory play a crucial role together with the patient-oriented public education campaigns. The most recent Clinical Practice Guidelines of the European Society of Cardiology on patients with acute myocardial infarction with ST-segment elevation (ESC STEMI Guidelines) were published in 2012 and covered the complexity of organisational and medical aspects from the emergent diagnosis and treatment until outpatient care.1 The reason for establishing the Stent for Life Initiative and its ACT NOW. SAVE A LIFE public campaign as a joint initiative of the European Association of percutaneous coronary intervention (EAPCI) and European Percutaneous Cardiovascular Revascularization Course (EuroPCR) was the lack of implementation of the Guidelines into the practice across Europe. The incredible achievements in 16 participating countries and organisations can be followed online at www.stentforlife.com and are well documented in the recent publication of Kristensen et al.2
Atypical electrocardiogram (ECG) presentations that deserve prompt management in patients with signs and symptoms of ongoing myocardial ischaemia include:
- left bundle branch block (LBBB);
- ventricular paced rhythm;
- patients without diagnostic ST-segment elevation but withpersistent ischaemic symptoms;
- isolated posterior myocardial infarction; and
- ST-segment elevation in augmented vector right lead (aVR).1
The patients with ongoing ischaemic symptoms, even in the absence of ST-segment elevations, should be managed in the same way as the STEMI patients. This recommendation, based more on experience than evidence, may shorten the delay to diagnosis and optimal treatment, and at the same time may decrease the risk of bleeding after the potent antithrombotic medication. A bit provocative is the suggestion of new and simplified classification of acute coronary syndrome with/ without ongoing myocardial ischaemia (ACS w/wo OMI) instead of the currently used STEMI and non-STEMI. Such an approach may better reflect current treatment practice in some catheterisation laboratories (cath labs) and regions with the aim of facilitating the decisions made at the time of the first medical contact (FMC). The target of such simplification is the earliest selection of patients at high clinical risk that should be transported directly to the cath lab or 24/7 primary percutaneous coronary intervention (PCI) centre bypassing any other facility. Widimsky et al.3 defined the ACS w OMI as ongoing (or recurrent) clinical signs of acute myocardial ischaemia (i.e. persistent chest pain and/or dyspnoea at rest) plus at least one of the following:
- ST-segment elevations in ≥2 consecutive ECG leads (≥2 mm for leads V2–V3, ≥0.5 mm for leads V7–V9 and ≥1 mm for other leads);
- new onset bundle branch block (right or left);
- persistent ST-segment depressions in ≥2 consecutive ECG leads (≥2 mm for chest leads and ≥1 mm for extremity leads);
- cardiogenic shock or ‘pre-shock’ type of haemodynamicinstability (low-to-normal blood pressure as well as tachycardia and cool extremities) due to suspected ischaemia;
- malignant arrhythmias including resuscitated cardiac arrest with return of spontaneous circulation;
- clinical signs of acute heart failure (Killip II–IV); and
- new onset of a wall motion abnormality on cardiac imaging.
It is important to keep in mind that isolated findings as listed above (e.g. malignant arrhythmias without any clinical or ECG sign of acuteischaemia) do not fulfil this definition. The high clinical suspicion for acute myocardial infarction is important. Direct transport to the cath lab (bypassing any other location, e.g. intensive care unit or emergency room) is always required in groups 1–4. Patients from groups 5–7 should also be transported to a non-stop (24/7) primary PCI facility (either directly to the cath lab or they may be primarily admitted to theintensive cardiac care unit with the cath lab immediately available). Healthcare systems with multiple organisational issues may especially benefit from such practical recommendations.
Time Intervals From Onset of Symptom Until Effective Treatment
The principle aim of the medical systems in patients with STEMI is to prevent their death and to improve the quality of life after a heart attack. Besides the effective treatment of ventricular tachycardia and fibrillation in the pre-hospital phase, shortening of the total ischaemic time as much as possible is crucial. ‘Patient delay’ and ‘system delay’, where the patient delay means the delay between symptom onset and FMC followed by the system delay until reperfusion therapy.
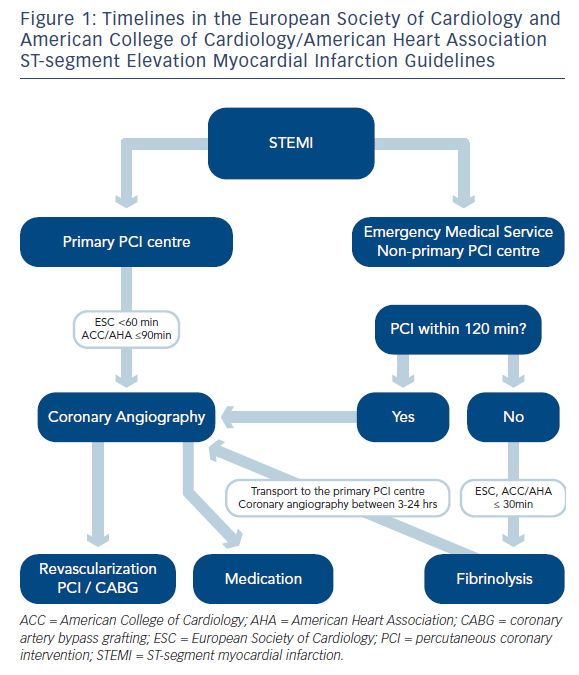
There is one important question with potential clinical consequences: who represents the FMC? Based on the ESC Guidelines, FMC is defined as the point at which the patient is either initially assessed by a paramedic or physician or other medical personnel in the pre-hospital setting, or the patient arrives at the hospital emergency department and therefore often in the outpatient setting.1 According to the time intervals mentioned in the ESC Guidelines, the preferred/ accepted system delay from FMC to the most effective reperfusion strategy (primary PCI, i.e. wire passage) should not exceed 90/120 minutes (min) in most patients and ≤60/90 min in high-risk patients with large anterior STEMI and early presenters within two hours. In case of fibrinolysis, the recommended system delay (FMC to needle) is ≤30 min. The diagnosis of STEMI should be verified within 10 min of FMC, which in fact means that the FMC personnel has to have the 12-lead ECG available. In future, the time of the medical system activation (i.e. the time of the first phone call) should be involved in the time intervals monitoring. The definition of the FMC might be modified to the first phone or personal contact of the patient with any representative of the medical system.
Current recommendation for reperfusion and the difference between the ESC and ACC/AHA4 STEMI Guidelines is described in Figure 1.
Primary Percutaneous Coronary Intervention – Procedural Aspects and Issues
Radial Over Femoral Access In Experienced Operators (Class IIa, Level of Evidence B)
The role of radial access has been investigated for 20 years5 but in STEMI patients this topic has been especially highlighted. The Radial Versus Femoral Access for Coronary Intervention (RIVAL) STEMI subanalysis6,7 (1,958 patients) and Radial Versus Femoral Investigation in ST Elevation Acute Coronary Syndrome (RIFLE-STEACS)8 trial (1,001 patients) demonstrated a significant mortality benefit in a large cohort of STEMI patients treated with primary PCI via transradial approach (TRA) (44 % reduction in all-cause death in RIVAL STEMI and 60 % in cardiac death in RIFLE-STEACS). These findings were supported by two large meta-analyses published recently by De Luca et al.9 and Karrowni et al.10 In the contrary, no survival benefit was found in the ST Elevation Myocardial Infarction Treated by RADIAL or Femoral Approach in a Multicenter Randomized Clinical Trial (STEMIRADIAL) in 707 patients.11 Although the direct association between the radial access and mortality may still be doubtful, the significantly lower risk of vascular and bleeding complications should promote the TRA as the preferred access site for most operators.12 Proper training and enough experience are necessary to achieve the optimal results that should receive the strongest level of evidence A in the upcoming guidelines.
Routine Manual Thrombus Aspiration (Class IIa, Level of Evidence B)
Recently, the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction study (TAPAS) was the only randomised trial showing the clinical benefit of routine thrombus aspiration versus conventional primary PCI. The mortality at oneyear, as the secondary and not pre-specified clinical endpoint, was found less frequently in the manual thrombus aspiration group (3.6 versus 6.7 %, p=0.018).13,14 The concerns about the single-centre experience and the technique of conventional PCI (balloon predilation before stenting) seem to be even more relevant after the first largescale international multicentre randomised Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia (TASTE) trial published by Fröbert et al.15 This trial reflects the real-world practice using a unique enrolment of patients from the national comprehensive Swedish Coronary Angiography and Angioplasty Registry (SCAAR registry) and endpoints evaluation through national registries without losing a single patient to follow-up. The clot aspiration in comparison with the balloon treatment in 7,244 STEMI patients did not show any statistical difference in mortality as the primary endpoint at 30 days (3.0 versus 2.8 %; p=0.63). This finding was consistent across all pre-specified subgroups and no difference was observed in therate of secondary endpoints obtained from the Swedish Websystem for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) registry and the national discharge registry (30-day rates of hospitalisation for recurrent myocardial infarction, stent thrombosis, target vessel revascularisation, target lesion revascularisation, and the composite of all-cause mortality or recurrent myocardialinfarction). Currently, the routine manual thrombus aspiration seems to be not supported by the evidence and should be used only selectively. The data from the ongoing Trial of Routine Aspiration Thrombectomy With Percutaneous Coronary Intervention (PCI) Versus PCI Alone in Patients With ST-Segment Elevation Myocardial Infarction (STEMI) Undergoing Primary PCI (TOTAL) with more than 10,000 patients (ClinicalTrials.gov: NCT01149044) will probably have a definite impact on the indication of routine thrombus aspiration during STEMI.16Periprocedural Pharmacotherapy
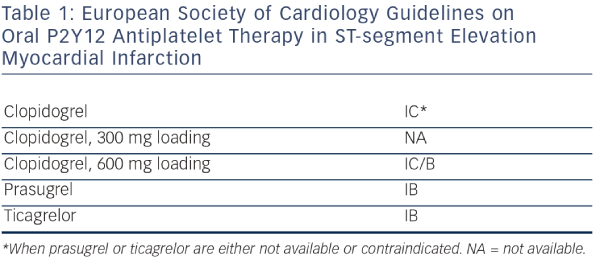
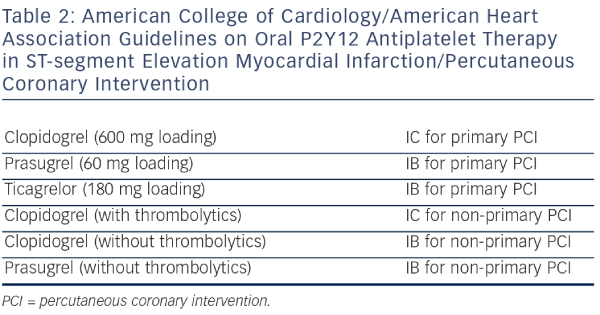
Based on the STEMI Guidelines, the dual antiplatelet therapy (Aspirin and an adenosine diphosphate [ADP] receptor blocker) is recommended together with parenteral anticoagulant as early as possible before angiography1 (i.e. immediately after the diagnosis of STEMI is confirmed). The novel agents, prasugrel or ticagrelor, should be preferred over clopidogrel17,18,19 in combination with bivalirudin,20 enoxaparin21 or unfractionated heparin.22 The use of glycoprotein IIb/IIIa inhibitors is indicated only as bailout in high-risk clinical situations, like the presence of large thrombus burden and no-flow phenomenon after PCI. The potential advantage of intracoronary administration of abciximab has been studied but the results have to be confirmed.23 Combination ofthe administration of potent drugs requires an individualised approach with respect to a patient´s risk profile (prothrombotic versus bleeding), complexity of coronary pathology, selected interventional strategy and a good clinical judgement.24 Recommendation of the ESC and ACC/AHA are shown in Tables 1 and 2.
Revascularisation Strategy for ST-segment Elevation Myocardial Infarction with Multivessel Disease
Culprit-only PCI has been recommended in STEMI patients except for patients in cardiogenic shock and continuous ischaemia despite successful infarct-related artery treatment.1 Recently, Wald et al. enrolled 465 patients with STEMI, multivessel disease and primary PCI of the infarct-related artery in the Randomized Trial of Preventive Angioplasty in Myocardial Infarction (PRAMI).25 The patients were randomly assigned to either preventive or no preventive PCI of the non-culprit vessels with at least 50 % stenoses. The study was stopped preliminary because of a highly significant decrease of the composite of death from cardiac causes, non-fatal myocardial infarction, or refractory angina in the preventive PCI group of patients (hazard ratio [HR] 0.35; p<0.001). Hazard ratios for the three components of the primary outcome were 0.34; 0.32 and 0.35, respectively. We are dealing with an exciting finding that needs further investigation. One of the unanswered questions in STEMI patients is the additional value of the functional revascularisation concept based on the fractional flow reserve measurement (ongoing Comparison Between Fractional Flow Reserve Guided Revascularization Versus Conventional Strategy in Acute STEMI Patients With multivessel disease [COMPARE-ACUTE] trial, ClinicalTrials.gov: NCT01399736).
Conclusion
Although the evidence-based approach described in the Guidelines may be applied to the majority of STEMI patients, the tailored and updated therapy needs to be modified in concordance with the patients´ risk profile, our experience and availability of medical resources. The establishment of the STEMI network and cooperation among all stakeholders can serve as the background for further improvements and new trials focused on clinical outcomes.





