Patients who undergo coronary artery stenting require dual antiplatelet therapy (DAPT) in order to reduce their risk of stent thrombosis. Long-term oral anticoagulation (OAC) is indicated for the primary and secondary prevention of thrombotic events in patients with atrial fibrillation (AF), mechanical heart valves, intra-cardiac thrombus, venous thromboembolic disease and some hypercoagulable states. Those patients who undergo percutaneous coronary intervention (PCI) and who have an indication for long-term OAC present a clinical dilemma in relation to their antithrombotic therapy – should they be treated with DAPT and OAC (‘triple therapy’), a regimen which is effective at the prevention of thrombotic events but which is associated with an increased risk of bleeding;1–4 should OAC be deferred in order to reduce the risk of bleeding but at the increased risk of systemic arterial or venous thrombotic events;5 or should the duration of DAPT be curtailed in order to reduce the risk of bleeding but with the potential increased risk of stent thrombosis? The advent of non-vitamin K antagonist oral anticoagulants (NOACs) and the increasing use of prasugrel and ticagrelor in patients who undergo PCI following an acute coronary syndrome (ACS) has further complicated clinical decision-making. This article aims to provide a summary of the evidence regarding antithrombotic therapy following PCI for patients who have an indication for OAC with a focus on recent randomised trials, which have influenced clinical practice in this area.
Antiplatelet Therapy to Prevent Coronary Artery Stent Thrombosis
Stent thrombosis is an infrequent but serious complication following PCI. Stent thrombosis within one month of PCI occurs with a frequency of 0.2–0.8 %, while stent thrombosis more than one month after PCI occurs at an approximate rate of 0.1–0.4 % per year.6 Stent thrombosis is associated with a mortality rate of up to 45 %.7 Platelet activation consequent to atheromatous plaque disruption during coronary angioplasty and stent deployment is one of the main mechanisms underlying stent thrombosis. Delayed stent strut epithelialisation and reactions to polymer are important mechanisms in cases of late drug-eluting stent (DES) thrombosis. Dual antiplatelet therapy is central to the prevention of stent thrombosis. Historical randomised trials showed a lower rate of stent thrombosis and of bleeding complications in patients who were treated with aspirin and ticlopidine than in patients who received aspirin and OAC.8–11 Ticlopidine use was associated with the common occurrence of side effects and blood dyscrasias, so aspirin and clopidogrel emerged as the standard post-PCI prophylaxis against stent thrombosis once trial data suggested equivalent efficacy for these two DAPT regimens.12 DAPT is required for four weeks after the deployment of a bare metal stent (BMS). Concerns regarding the incidence of late stent thrombosis in patients who were treated using first generation DES have largely dissipated with modifications in stent design and polymer technology.13 Nevertheless, it is usual for DAPT to be taken for at least 6–12 months after second or third generation DES use. Premature discontinuation of DAPT is the most consistent predictor of stent thrombosis.7
Oral Anticoagulation for Stroke Prevention in Patients with Atrial Fibrillation
Vitamin K Antagonists
AF is the most common indication for long-term OAC. AF affects up to 2 % of the general population and as many as 15 % of octogenarians.14–16 Blood stasis within the non-contractile left atrium predisposes these subjects to thromboembolic cerebral infarction, with an average annual stroke rate of about 5 % in non-valvular AF.17 OAC with a vitamin K antagonist (VKA) such as warfarin is highly effective at reducing the risk of stroke in patients with AF. In a meta-analysis of 29 studies, which included 28,044 patients, for example, VKA therapy was associated with a 67 % relative reduction in the rate of ischaemic stroke.18 Warfarin is substantially more effective than aspirin, which reduced the risk of stroke in patients with AF by 22 % in placebo-controlled trials.18,19 Warfarin is also considerably more effective than DAPT at preventing stroke in patients with AF. Atrial Fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE- W) was stopped early when a clear significant result was demonstrated between patients who were randomly allocated to VKA therapy compared with aspirin and clopidogrel, with an annual rate of stroke, systemic embolus, myocardial infarction (MI) or vascular death of 3.9 % in the VKA group compared with 5.6 % in the DAPT group.5 ACTIVE-A subsequently showed that aspirin and clopidogrel was superior to aspirin alone for stroke prevention in patients with AF with a relative risk reduction of 28 %.20 Discontinuation of OAC in patients with AF, even temporarily, is associated with an increased stroke risk.5
Non-vitamin K Antagonist Oral Anticoagulants
NOACs such as the direct thrombin inhibitor, dabigatran and the factor Xa inhibitors, rivaroxaban and apixaban, have recently emerged as alternatives to warfarin for stroke prevention in patients with AF. When compared with a VKA in randomised trials, dabigatran 150 mg twice daily reduced the risk of stroke,20 dabigatran 110 mg twice daily reduced the rate of major bleeding,20 apixaban reduced the rate of mortality, stroke and major bleeding,21 and all three agents were associated with a lower rate of intracerebral haemorrhage.21–23 Furthermore, their use has practical advantages, which include fixed dosing, no need for blood test monitoring and fewer drug interactions when compared with warfarin.
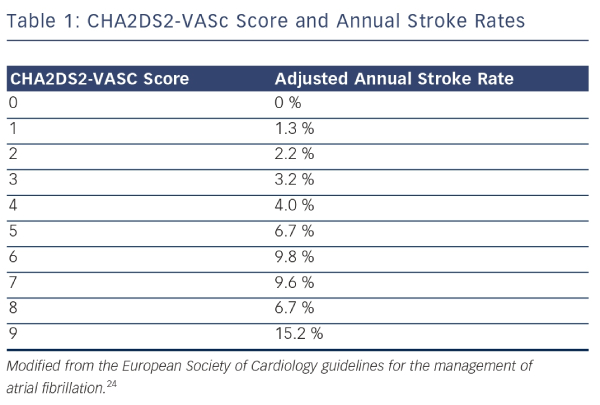
These data form the evidence base for current clinical practice regarding stroke prevention in patients with AF. The balance between benefit and risk from OAC should be assessed through formal stratification of stroke risk using the CHA2DS2-VASc score (see Table 1) and the bleeding risk using the HAS-BLED score.24,25 Stroke risk is very low in men with a CHA2DS2-VASC score of 0 and in women with a CHA2DS2- VASc score of 1. A HAS-BLED score ≥3 is associated with an elevated risk of bleeding in patients taking OAC. Nevertheless, most patients with AF should be treated with OAC to lower their risk of stroke.24 Aspirin monotherapy is not recommended for the prevention of stroke in patients with AF.26 DAPT should only be considered for this purpose in patients who refuse to take OAC or in whom there is a clear contraindication to OAC and the bleeding risk is low.24
Antithrombotic Therapy Following Percutaneous Coronary Intervention for Patients with an Indication for Oral Anticoagulation
Nearly 10 % of patients who undergo PCI have an indication for longterm OAC.27 Coronary artery disease which requires PCI is present in more than 20 % of patients with AF.28 There are currently no definitive randomised trial data to guide decision-making regarding antithrombotic regimens following PCI for these patients. The inferior efficacy of OAC compared with DAPT for the prevention of stent thrombosis and the converse situation in relation to stroke prevention in patients with AF has led to the assumption that all three antithrombotic agents are required in order to achieve an acceptably low rate of adverse cardiac and cerebral events after PCI in patients who have AF.
Triple Antithrombotic Therapy – Rates of Vascular Events and Bleeding
The combination of all three antithrombotic agents after PCI might be expected to minimise the risk of both stroke and stent thrombosis but it would also be expected to increase bleeding rates. Data from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association guidelines (CRUSADE) registry showed that approximately one-third of patients with ACS who were established on OAC at hospital admission had their OAC discontinued when DAPT was started following PCI.29 It seems likely that this practice related to concerns regarding the risk of bleeding with triple therapy. In the absence of randomised trials in this area, information regarding clinical outcomes for patients who have received triple therapy come from observational studies. A recent meta-analysis of nine studies, which included 1,996 patients requiring OAC who underwent PCI, compared clinical outcomes in those who received triple therapy with those who received DAPT.30 In this analysis, triple therapy was associated with a lower rate of cardiac death, MI, stent thrombosis or target lesion revascularisation (odds ratio [OR] 0.60; 95 % confidence interval [CI] 0.42–0.86; p=0.005) and all-cause mortality (OR 0.59; 95 % CI 0.39–0.90; p=0.01) compared with DAPT. Major bleeding in the first six months after PCI, however, was significantly more common (4.1 versus 1.9 %; p=0.04) in patients who received triple therapy than DAPT (OR 2.12; 95 % CI 1.05–4.29).
In registries of patients who received triple therapy after PCI, the rate of major bleeding or need for blood transfusion ranged from approximately 5 % early after PCI to more than 10 % at 12 months.1–4 In a nationwide Danish registry of 11,480 patients with AF who were admitted for MI or PCI between 2001 and 2009, bleeding requiring hospital admission was significantly more frequent (14.2 events per 100 person-years) in patients who received triple therapy than in patients who received DAPT or OAC plus either aspirin or clopidogrel (9.7 versus 7.0 versus 6.9 events per 100 person-years, respectively).31 Furthermore, the bleeding risk was front-loaded with a hospital admission rate higher than 20 % in the first 90 days after PCI in patients who received triple therapy. In the post hoc analysis of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) study, the rate of major bleeding after PCI for acute MI during the index hospital admission was 17.1 % in patients treated with triple therapy compared with 6.5 % in patients who received DAPT (p<0.0001).32 Major bleeding or the need for blood transfusion after PCI is associated with increased mortality.33 Minor bleeding complicates clinical management and may lead to discontinuation of OAC or DAPT with consequent loss of their protective effect against thrombotic vascular events.5,7 It is desirable to limit the duration of triple therapy as much as possible, particularly in patients who are at high risk of bleeding. In patients who require OAC, this can only be achieved by curtailing the period of DAPT.
Limiting the Duration of Dual Antiplatelet Therapy After Percutaneous Coronary Intervention
BMS use affords the opportunity to limit the duration of triple therapy to one month after PCI in patients who require OAC. However, there are well-founded reasons why DES use might be preferable to BMS use in some patients who require OAC. The use of DES more than halves the requirement for repeat revascularisation compared with BMS use. Minimising the requirement for repeat invasive procedures is particularly desirable in patients who are taking OAC. Those patients who are at high risk of restenosis are likely to benefit most from DES use.
Several randomised controlled trials have now been published, which compared clinical outcomes in patients who received limited duration or standard duration DAPT after PCI using DES. In the REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation (RESET) trial, 2,117 patients who underwent PCI using the Endeavor® zotarolimus eluting stent (ZES) (Medtronic Inc, Minneapolis, Minnesota, US) were randomly assigned to receive either three months or 12 months DAPT after the procedure.34 The primary endpoint of cardiovascular death, MI, stent thrombosis, target vessel revascularisation (TVR) or bleeding at one-year occurred in 4.7 % patients in each group (p<0.0001 for non-inferiority of three months compared with 12 months DAPT). The rates of death, MI or stent thrombosis were 0.8 versus 1.3 % (p=0.48) and of stent thrombosis were 0.2 versus 0.3 % (p=0.65), respectively, in patients who received three months compared with 12 months DAPT. There were no cases of stent thrombosis after three months in the limited duration DAPT group. The Optimized duration of Clopidogrel Therapy Following Treatment with the Zotarolimus-Eluting Stent in Real- World Clinical Practice (OPTIMIZE) trial was of similar design, involving the random allocation of more than 3,000 patients undergoing PCI with ZES to receive either three months or 12 months DAPT.35 At one-year the rate of the primary endpoint (a composite of all-cause mortality, MI, stroke and major bleeding) was 6.0 % in the three- month DAPT group compared with 5.8 % in the 12-month DAPT group (p=0.002 for non- inferiority of three months versus 12 months DAPT). One-year rates of major adverse cardiac events were similar between groups (8.3 versus 7.4 %, respectively; hazard ratio [HR] 1.12; 95 % CI 0.87–1.45). Nor were there significant differences between groups in the rates of the primary net clinical benefit endpoint, major adverse cardiac events or stent thrombosis that occurred between three and 12 months.
The Resolute ZES (Medtronic Inc, Minneapolis, Minnesota, US) has CE Mark approval for only one month of DAPT following deployment. This was granted after analysis of a cohort of 4,896 patients who received this stent showed that the rate of stent thrombosis was very low (0.11 %) among the 903 patients whose DAPT was interrupted more than one month (and less than 12 months) following PCI.36 This rate was no higher than the 0.84 % rate of stent thrombosis observed among the 3,827 patients whose DAPT was uninterrupted, and it was significantly lower than the rate of 3.61 % in the 166 patients whose DAPT was interrupted within one month of stent deployment (p<0.001). In this cohort, DAPT was interrupted for >14 days in 874 patients. All six cases of stent thrombosis occurred in the 122 patients whose DAPT was interrupted within the first month of therapy while there were no cases of stent thrombosis among the 752 patients whose DAPT was interrupted between one and 12 months after PCI. The rate of cardiac death or target vessel MI was 6.84 % among patients whose DAPT was interrupted for >14 days within one month of PCI, 1.41 % when DAPT was interrupted for >14 days between one and 12 months after PCI and 4.08 % in the 4,071 patients whose DAPT was not interrupted for >14 days.
Is Dual Antiplatelet Therapy Necessary After Percutaneous Coronary Intervention in Patients Taking Oral Anticoagulation?
The question of whether or not DAPT (and thereby triple antithrombotic therapy in patients who require OAC) is needed at all following PCI was investigated in the What is the Optimal antiplatElet therapy in patients with oral anticoagulation and coronary StenTing (WOEST) trial.37 In this trial, 573 patients who were receiving OAC with a VKA and who underwent PCI were randomly assigned to clopidogrel and OAC or to triple therapy. The primary endpoint of any bleeding event within one year of PCI occurred significantly less frequently in patients in the clopidogrel and OAC group compared with the triple therapy group (19.4 versus 44.4 %; HR 0.36 [95 % CI 0.26–0.50]; p<0.0001). The secondary endpoint, a composite of death, MI, stent thrombosis, stroke or TVR, was also significantly reduced in patients taking clopidogrel and OAC compared with triple therapy (11.1 versus 17.6 %; HR 0.60 [95 % CI 0.38–0.94]; p=0.025). The omission of aspirin therefore led to a greater than 50 % reduction in bleeding without a detectable increase in adverse cardiovascular events. These results suggest that triple therapy may be unnecessary following PCI for patients who are treated with OAC.
Before applying the findings of the WOEST trial to routine clinical practice, several observations should be made about its methodology and results. The trial medication was administered in open label fashion, which might have introduced bias into the results. Most of the bleeding episodes were ‘minor’ or ‘moderate’ in severity depending upon the bleeding event classification used. There were relatively low rates of radial artery access and proton pump inhibitor use, which might have mitigated against lower bleeding rates. Importantly, the WOEST trial was not adequately powered to demonstrate non-inferiority for the composite secondary endpoint of cardiovascular events. On the other hand, the bleeding events committee were blinded to treatment allocation; the difference in bleeding events between groups was accounted for by lower rates in the clopidogrel and OAC group of overt haemorrhage causing a drop in haemoglobin concentration of 3–5 g/dL or a drop in haemoglobin concentration of >4 g/dL without overt haemorrhage (Thrombolysis In Myocardial Infarction [TIMI] minor bleeding) and by a lower rate of blood transfusions (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries [GUSTO] study moderate bleeding), events well worth avoiding; and there was no trend towards higher rates of stent thrombosis in patients who received clopidogrel and OAC compared with triple therapy, with rates of any stent thrombosis of 1.4 versus 3.2 %, definite stent thrombosis 0.4 versus 1.1 %, probable stent thrombosis 0 versus 0.7 % and possible stent thrombosis 1.1 versus 1.4 %, respectively. Indeed, the significantly lower rate of cardiovascular events in the clopidogrel and OAC group makes it unlikely that a clinically meaningful excess of cardiovascular events arises from omitting aspirin after PCI in patients who are taking clopidogrel and OAC. Furthermore, these findings are consistent with an analysis of 12,165 patients with AF hospitalised for MI or PCI in the Danish registry referred to earlier in which one-year rates of cardiovascular events and all-cause mortality were compared in patients who received different antithrombotic regimens.38 Rates of MI or cardiovascular death, stroke and all-cause mortality were similar in patients who were treated with the combination of clopidogrel and OAC with a VKA compared with triple therapy. By contrast, the ischaemic stroke rate was significantly higher in patients who were treated with DAPT (aspirin and clopidogrel) and all-cause mortality was significantly higher in patients who were treated with DAPT or with aspirin and OAC. Taken together, these data suggest that clopidogrel and OAC is at least as effective and as safe as triple therapy.
Potential Future Antithrombotic Strategies with a Limited Evidence Base
Non-vitamin K Antagonist Oral Anticoagulant-based Antithrombotic Therapy
Dabigatran, apixaban and rivaroxaban were associated with significantly lower rates of intracranial haemorrhage than warfarin in randomised trials of stroke prevention in patients with AF,21–23 while dabigatran at a dose of 110 mg twice daily22 and apixaban also significantly reduced the rate of major bleeding compared with VKA.21 These data combined with the more predictable pharmacokinetic and pharmacodynamic profile of NOACs compared with VKAs raise the prospect of triple therapy or dual therapy (NOAC and clopidogrel) regimens, which carry a lower risk of bleeding than warfarin-containing regimens.
Insights into the risk of bleeding with NOAC-based triple therapy come from studies, which have investigated the combination of a NOAC with DAPT to reduce ischaemic cardiac events in patients following ACS. In the Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome - Thrombolysis In Myocardial Infarction (ATLAS ACS 2-TIMI 51) study, the addition of low dose rivaroxaban (either 2.5 mg or 5 mg twice daily) to DAPT reduced the composite endpoint of cardiac death, MI and stroke after ACS but increased the rate of major bleeding and of intracranial bleeding.39 The ATLAS ACS 2-TIMI 46 study was designed to assess the safety and efficacy of various doses of rivaroxaban in combination with DAPT after ACS. In this study, the combination of DAPT with rivaroxaban 20 mg daily, its usual dose for stroke prevention in AF, rates of TIMI major and TIMI minor bleeding within six months were low at 1.8 and 0.9 %, respectively, but the rate of bleeding requiring medical attention at 180 days was 14.3 %.40 In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RELY) study, which defined the relative efficacy and safety of dabigatran 110 mg twice daily, dabigatran 150 mg twice daily and warfarin for stroke prevention in AF, 38 % of the 18,113 patients also received antiplatelet therapy during the study period. The rate of bleeding was significantly higher in patients who took OAC plus one antiplatelet agent than in patients who took OAC only and was highest in patients who took OAC and DAPT irrespective of the OAC used. However, the rate of bleeding was lowest for patients who took dabigatran 110 mg twice daily, whether they were taking OAC only, OAC plus one antiplatelet agent or OAC plus DAPT.41 NOAC-based triple therapy has not yet been compared with warfarin-based triple therapy in a randomised trial. Further research is needed to define whether or not NOAC or warfarin is the preferred OAC for patients who require OAC following PCI.
Antithrombotic Combination Therapy Incorporating New Antiplatelet Agents
About 20 % of patients who undergo PCI achieve inadequate inhibition of platelet function from clopidogrel therapy.42 These clopidogrel non-responders experience higher rates of cardiovascular events than clopidogrel responders. Prasugrel and ticagrelor are newer, more potent antiplatelet agents than clopidogrel, which are being used with increasing frequency after ACS.
Prasugrel is a potent thienopyridine, which has less inter-individual variation in platelet inhibition than clopidogrel. In the Trial to Assess Improvements in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel - Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38), prasugrel and aspirin was superior to clopidogrel and aspirin in reducing adverse cardiac events (primarily MI and stent thrombosis) following an ACS.43 The increased efficacy of prasugrel came at the expense of significantly increased rates of major bleeding, including life-threatening bleeding. In the only published registry of patients taking triple therapy comprising aspirin, prasugrel and warfarin (used in 21 patients), this regimen was associated with a more than fourfold increase in bleeding (28.6 versus 6.7 %; p<0.001) without any difference in efficacy when compared with aspirin, clopidogrel and warfarin (taken in 356 patients).44
Ticagrelor is a direct-acting, reversible inhibitor of the platelet P2Y12 receptor. Like prasugrel, it provides more consistent inhibition of platelet function than clopidogrel. In the Study of Platelet Inhibition and Patient Outcomes (PLATO), patients with ACS who were randomly allocated to receive the combination of ticagrelor and aspirin experienced lower rates of recurrent MI and of all-cause mortality than patients who were randomly allocated to receive clopidogrel and aspirin.45 There was no significant difference in rates of major bleeding between the ticagrelor and clopidogrel groups (11.6 versus 11.2 %, respectively; p=0.4). There are currently no data, which describe the efficacy and safety of ticagrelor-based triple therapy. In PLATO, for instance, the study medication was stopped if OAC was administered. However, a recently published small Swedish study described outcomes among 107 patients with ACS who were discharged taking a combination of ticagrelor and warfarin and compared them to a historical control group of 159 patients with a similar baseline bleeding risk who were discharged taking aspirin, clopidogrel and warfarin.46 Rates of major bleeding and of ischaemic events (a composite of stroke or TIA, arterial embolism and recurrent ACS) at three months were not significantly different (7.5 versus 7.0 % and 4.7 versus 3.2 %, respectively) in patients taking ticagrelor and warfarin compared with triple therapy. Further data are required before firm recommendations can be made regarding combinations of antithrombotic therapy, which include OAC and prasugrel or ticagrelor.
Current Guidelines
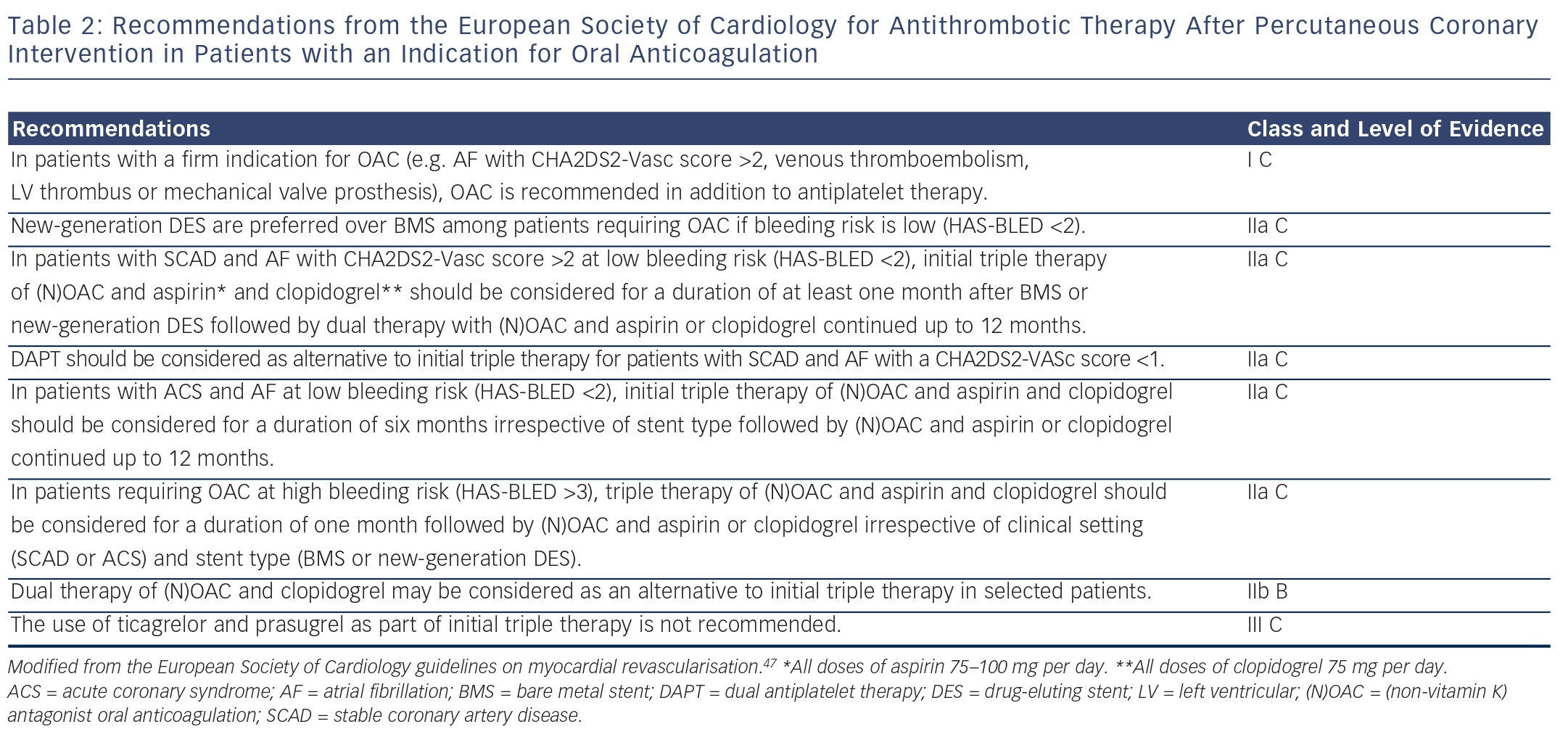
The use of triple therapy in patients after PCI is determined by the strength of their indication for OAC. The 2014 European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines on myocardial revascularisation recommend that triple therapy should be used in patients with AF who have a CHA2DS2-VASc score >2, mechanical heart valves, intracardiac thrombus or recent or recurrent venous thromboembolism (see Table 2).47 They recommend that decisions regarding stent type (DES or BMS) and duration of triple therapy are made after formal assessment of the bleeding risk using the HAS-BLED score. Patients who are at low risk of bleeding (HAS-BLED score ≤2) should be treated using new-generation DES in preference to BMS, since these patients are likely to tolerate a longer period of triple therapy than other patients. By contrast, it is recommended that the duration of triple therapy should not exceed one month in patients who have a high bleeding risk (HAS-BLED score >3). In these patients, the choice of stent (DES or BMS) should be decided on an individual basis, and OAC and clopidogrel may be considered as an alternative to triple therapy.
Conclusion
The optimal antithrombotic regimen following PCI in patients who require OAC is yet to be determined. The different balance in individual patients between their risk of arterial or venous thrombotic events, stent thrombosis and bleeding means that there is unlikely to be a single antithrombotic regimen of choice for all patients who have an indication for OAC after PCI. Current guidelines recommend triple therapy comprising aspirin, clopidogrel and either warfarin or a NOAC in the post-procedure period for all patients who have a clear indication for OAC in order to reduce their risk of stent thrombosis and thrombotic arterial or venous events. However, this regimen is associated with a high rate of bleeding complications. Recently published trials suggest that DAPT (and thereby triple therapy in patients who require OAC) can be limited safely to three months, and potentially to one month, following the deployment of new-generation DES without an excess risk of stent thrombosis. Furthermore, triple therapy may be unnecessary, the combination of OAC plus clopidogrel (without aspirin) providing similar protection against stroke and stent thrombosis but with a significant reduction in bleeding compared with triple therapy in one randomised trial.