The transradial approach (TRA) was introduced for the first time in 1989.1 Used at first by Campeau for coronary angiography, the TRA was later applied by Kiemeneij et al. for percutaneous coronary intervention (PCI).2 The radial artery has proven to be a challenging but safe route towards the coronary arteries. According to the European Society of Cardiology (ESC) guidelines, the radial artery should be the preferred route for percutaneous coronary procedures in acute coronary syndrome (ACS) patients.3 However, the transfemoral approach (TFA) is still used in many centres for primary PCI, mostly in fear of longer door-to-balloon times and worse procedural outcomes.4 In our centre, the TRA represents the first choice treatment in cases of primary PCI. In this review, we will first present the published evidences, then we will share the data and experiences at Onze Lieve Vrouwe Gasthuis (OLVG) Amsterdam, and finally we will discuss the issues of radial artery occlusion and indications for the TFA at a TRA centre.
Transradial Versus Transfemoral Approach in ST-elevation Myocardial Infarction
According to both European and North American guidelines, primary PCI is the recommended treatment in patients with ST-elevation myocardial infarction (STEMI), if it can be performed within 2 hours.5,6 Primary PCI provides a higher and faster rate of coronary reperfusion than thrombolysis. Unfortunately, the risk of bleeding complications is not solved. Primary PCI-related bleedings are due to the use of multiple antithrombotic and antiplatelet agents, which is unavoidable to prevent recurrent ischaemic events. The occurrence of a bleeding is not without risk as it is associated with higher mortality.7,8,9 The relationship between bleedings and mortality is often multifactorial, e.g. haemodynamic compromise, discontinuation of antithrombotic agents and prothrombotic state. Although non-access site bleedings bear a greater relative risk than access site-related bleedings, the latter are also potentially life-threatening. Verheugt et al. showed that, in ACS patients undergoing PCI, an access site bleeding is associated with a twofold increase in 1-year mortality, whereas a non-access site bleeding is associated with a fourfold increase.8 These findings underline the urge not only to refine our therapies (fast on/ off antithrombotics, such as bivalirudin, choice of antiplatelet agents according to bleeding risk score), but also the way interventional procedures should be performed to minimise the risk of bleedings.
The first study to compare both femoral, brachial and radial access for PCI was performed by Kiemeneij et al.10 The failure rate of PCI was equal between all groups (8.3, 9.0 and 8.6 %, respectively), and no difference in major adverse cardiac events at 1-month was observed. However, there was a reduction in entry site-related bleeding complications (0.0, 2.3 and 2.0 %, respectively for radial, brachial and femoral access; p=0.035). This pivotal study did not comprise acute patients.
The Radial Versus Femoral Access for Coronary Intervention (RIVAL) trial was the first, large-scale multicentre randomised trial comparing the TRA and the TFA in ACS. In this study 7,021 patients undergoing coronary angiography and intervention because of ACS were included.11 The authors detected similar results: no difference in major adverse cardiac events (3.7 and 4.0 %, respectively) but less bleeding in the TRA group (1.9 and 4.5 % Acute Catheterization and Urgent Intervention Triage Strategy [ACUITY] bleeding, respectively) and less access site complications (1.4 and 3.7 %, respectively). A sub-analysis of patients with STEMI showed a lower incidence of major adverse cardiac events when the TRA was used compared with the TFA (2.7 and 4.6 %, respectively; p=0.031). Door-to-balloon times were comparable between the TRA and the TFA, as it is confirmed by other studies.12,13
An additional meta-analysis, including all studies comparing the TRA and the TFA PCI in ACS, showed a significant reduction in major bleeding (0.9 versus 1.6 %, respectively), and also a reduced mortality (1.8 versus 2.5 %, respectively), when the TRA was used.14
The Radial Versus Femoral Randomized Investigation in ST-segment Elevation Acute Coronary Syndrome (RIFLE-STEACS) trial, specifically comparing the TRA and the TFA for primary PCI, showed lower mortality with the TRA than the TFA (7.2 versus 9.2 %, respectively; p=0.02).12 The incidence of bleeding, especially access site-related bleeding, was higher in the TFA group. This concomitant reduction corroborates the link between mortality and bleeding. Finally, a recent metaanalysis, pooling 16 randomised studies, showed a relative risk reduction of 33 % in mortality when the TRA is used and 48 % reduction in major bleeding.15
The Minimizing Adverse Haemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox (MATRIX) study is a large trial in which 8,404 ACS patients undergoing PCI were randomised to either the TRA or the TFA. Patients undergoing the TRA had significantly less net adverse clinical events (9.8 %) compared with patients with femoral access (11.7 %). This difference was driven by major bleeding unrelated to coronary artery bypass graft surgery (1.6 versus 2.3 %, respectively for the TRA and the TFA), but also by all-cause mortality (1.6 versus 2.2 %, respectively for the TRA and the TFA).16 A peculiarity of the MATRIX study is that it was conducted by experienced radial operators only.
Transradial Primary Percutaneous Coronary Intervention at Onze Lieve Vrouwe Gasthuis Amsterdam
Pre-procedural Logistics
OLVG Amsterdam is a large non-academic tertiary referral centre in the centre of Amsterdam, where about 3,000 percutaneous coronary procedures are performed each year, among which about 300 primary PCI and 500 PCI for ACS are performed. The great Amsterdam region consists of an area with 1.4 million inhabitants with three PCI centres and five non-PCI community hospitals. Catchment areas, with active back-up between hospitals, are defined according to postal codes. Patients are directly referred by the ambulance team to the catheter laboratory of the appropriate PCI centre, after teleconsultation (including electrocardiogram).
If the patient is accepted for primary PCI, he/she will receive an upload dose of antiplatelets and antithrombotics (Ascal® 160 mg, Ticagrelor 180 mg and Heparin 5,000 UI) in the ambulance. In particular, the choice for Ticagrelor, instead of other P2Y12 inhibitors (Clopidogrel, Prasugrel), has been based both on its efficacy and safety profile in cases of primary PCI and on its broad spectrum of use in ACS patients.17 Thanks to our system, which bypasses both community hospitals and the emergency department, first medical contact-to-balloon time is short – around 82 minutes (interquartile range 70–98).18
Procedural Results
At OLVG Amsterdam, the TRA is the standard access site for primary PCI. This is our first treatment choice due to the expectation that clinical outcomes would be better, and to speed up patient turn-over as well as allow faster PCI access to more patients.
Vink et al. reported on the procedural outcomes of the TRA primary PCI in our institution between the years 2001 and 2008.19 In this timeframe, 2,484 primary PCI were performed, of which 184 procedures were excluded from analysis because of cardiogenic shock. In the remaining 2,300 cases, the TFA was chosen by the operator in 91 cases (3.9 %), mainly because of recent TRA (19.7 %) or anatomic impediments (18.6 %). Crossover occurred in 3.8 %, mainly because of the inability to reach the coronary ostium (35.7 %) or due to inadequate support of the guiding catheter (16.7 %). Over the years, the need for crossover decreased from 5.9 % to 1.5 %. Procedural success remained stable over time, around 94 %, while procedural times decreased from 38 to 24 minutes. These findings can be attributed to better patient selection, improved materials (in particular puncture kits), but also growing operator experience.
These results were also found in the RIVAL trial, where crossover was lower in high-volume centres (4.4 %).11 Indeed, even for OLVG operators, who according to the RIVAL trial definition (>146 cases using the TRA per year) would be defined throughout the years as high-volume operators, there is a continuous margin for improvement, which highlights the technical challenges of the TRA. As a result of the small diameter and anatomic variations of the radial artery, the TRA is more demanding than the TFA. Therefore, the TRA requires a longer learning curve for the operator.20 More experienced operators have lower procedure times, equalling or outperforming the TFA.21 The same applies to radiation exposure.22 Overall, fluoroscopy time is longer and radiation burden is higher with the TRA, except in TRA experienced operators. Also, in our experience radiation exposure is not higher for radial versus femoral procedures, but it is for fellow versus staff interventional cardiologists.
To evaluate the impact of a systematic TRA in primary PCI on clinical outcomes, we performed a retrospective analysis of all the primary PCIs of 2015 (unpublished data). The study cohort consisted of 194 patients, directly admitted to the catheter laboratory from the ambulance service, with exclusion of self-referrals to the emergency department and cardiogenic shock.18 The TFA was used in only 3.6 % of patients. No serious bleeding events occurred. The in-hospital mortality was 5.7 % and cumulative 30-day mortality was 6.2 %, which is somewhat worse than results from highly selected randomised studies, such as the Administration of Ticagrelor in the Cath Lab or in the Ambulance for New St-elevation Myocardial Infarction to Open the Coronary Artery (ATLANTIC) study, but comparable to other STEMI reports.12,23 The median door-to-needle time was 21 minutes (interquartile range 14–38) and the median door-to-balloon time was 39 minutes (interquartile range 27–60). This is within the set limit of 60 minutes.3 Based on the published studies and our own experience we are convinced that the TRA guarantees excellent procedural outcomes in cases of primary PCI as well as favourable clinical results.
These data collectively suggest the need for the routine use of the TRA percutaneous coronary procedures, as procedure success increases with the experience of the operator, resulting in less catheter manipulation and exchange, and reduces radial artery spasm.
Technical Considerations
Procedural techniques for engaging the coronary arteries and performing interventions can vary according to the operator’s preferences and experience. Despite the fact that the right subclavian and brachiocephalic arteries will present slightly more abnormalities than the left ones, all operators at OLVG Amsterdam choose the right radial artery as first access (96 % of primary PCI procedures). This is mostly due to the table set-up and operator’s comfort. When the left radial artery is punctured (i.e. either the right radial is not feasible, or a need to film the left mammary artery), the operator will puncture from the left side of the patient and then the arm will be bent on the abdomen of the patient to be able to continue the procedure from the right side of the table. In our experience, there is no significant difference in procedural and clinical outcomes for primary PCIs performed either from the right or left side.
There is some agreement that a single catheter strategy will prevent manipulation and radial spasm and is therefore pursued in most of the primary PCI procedures, by using curves which are suitable for both left and right ostia (Multipurpose, Radial, Kimny, Judkins Left). The expected non-culprit vessel is filmed first, to assess any collateral filling and/or additional atherosclerotic burden. Thromboaspiration is performed on indication (Thrombolysis In Myocardial Infarction [TIMI] flow 0-1 or evident thrombotic filling defect). Most STEMI patients will receive a limus-eluting stent and be either transferred to peripheral hospital directly after the procedure or remain admitted for 3 days after a successful PCI. Tricks learnt in the elective setting to overcome the sometimes challenging anatomy of the radial artery are very useful before approaching the TRA primary PCI; for instance, balloonguided tracking of the guiding catheter through a radial loop or how to manage subclavian tortuosity. The use of dedicated TRA guiding catheters and/or improvement in handling skills would eventually lead to higher success rates. The TRA expertise accumulated in complex elective procedures will warrant comparable procedural times and success rates in the acute setting. Practice makes, once more, perfect.
Post-procedural Logistics
After the TRA, post-procedural bed rest is not necessary, permitting immediate ambulation. This is more comfortable for the patient and reduces the workload of the staff.24 Moreover, prolonged bed rest itself seems to be a predictor of a worse prognosis in coronary artery disease.25 As the TRA enables early discharge, it is associated with a significant reduction in duration of hospital stay and costs.26 These advantages are, of course, more evident in the elective setting, where the TRA allows day care PCI. At OLVG Amsterdam most of the elective TRA PCIs are allowed to go home the same day, provided that the procedural result has been successful and that no complications occur in the first 6 hours after the procedure. In a survey of 397 patients who had undergone an uneventful elective TRA PCI in 2014, 387 of the 397 patients (97.5 %) were allowed to go home the same day, while 10 of the 397 patients (2.5 %) remained in the hospital either because of minor bleedings or suspected angina complaints (unpublished data). Whereas patients who undergo a primary PCI cannot be discharged home on the same day, patients after an uneventful TRA primary PCI can immediately be transferred to a community hospital for surveillance, making the catheter laboratory and coronary care unit available for the following patients. Time for observation can be kept short, as the risk of access site complications is low after the TRA.11 This strategy is of increasing importance, as recent European guidelines dictate that most patients with ACS should undergo coronary angiography in a PCI-capable centre, within 24 hours of hospital admittance.27 At OLVG Amsterdam, patients are usually transferred to community hospitals 2 hours after a successful TRA primary PCI. Only patients living in the neighbourhood (East and Central Amsterdam: 20–30 %) will remain at OLVG and, also thanks to fast mobilisation, will be discharged home after two or three days. By doing this, the TRA allows us high efficiency and best clinical practices at the same time.
The Issue of Radial Artery Occlusion
As a result of its superficial course, the radial artery can be easily punctured and compressed to obtain haemostasis. While serious bleeding complications are uncommon, the TRA bears the risk of radial artery occlusion (RAO). The incidence of RAO varies between 3 % and 10 %, according to different studies and protocols.28,29
RAO rarely results in serious adverse events nor is it symptomatic, but excludes a TRA for future procedures.30 Therefore, any effort should be taken to reduce the risk of RAO.
At OLVG Amsterdam, Heparin 5,000 IU is given after sheath insertion during every TRA procedure to retain patency.31 During compression we will also take care that the artery does not get completely occluded and that flow in the radial artery is maintained (‘patent haemostasis‘). With patent haemostasis the incidence of RAO has been shown to decrease by 75.0 % to 1.8 %.32
Finally, the choice of an appropriate sheath size is fundamental. Mismatch between inner diameter of the radial artery and outer diameter of the introducer can cause vascular trauma. A percentage of patients ranging from 14 % to 27 % have a radial artery that is smaller than a 6-French introducer.33 Larger sheath diameter increases the risk of RAO.34
To avoid mismatch between radial artery and introducer, the TRA PCI techniques have been developed with the use of miniaturised catheters and materials, so-called Slender PCI. Both the Slender Club Japan and Europe focus on maximal miniaturisation of the TRA PCI. By using 5-French introducers the chance that the radial artery would be smaller than the introducer is reduced by 50 %.33 In cases where 5-French TRA PCI are performed without the introducer (so-called ’5-French sheathless PCI‘, or ’virtual 3-French PCI‘), the chance to find a radial artery smaller than the introducer is close to 0 %.35 Slender TRA PCIs have shown to be feasible, safe and effective in a variety of elective settings, ranging from bifurcation stenting to straightforward chronic total occlusion.35,36,37
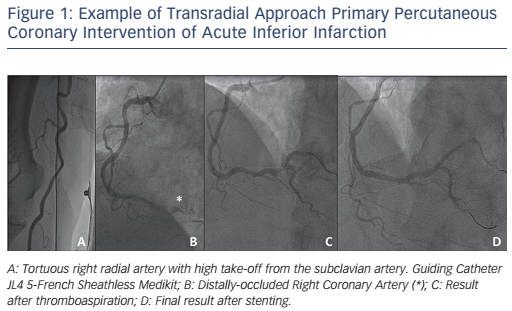
The use of Slender TRA techniques for primary PCI had been at first limited by the availability of compatible materials, in particular thrombus-aspiration catheters, which were until recently only compatible with 6-French or larger guidings. The results of the Randomized Trial of Routine Aspiration Thrombectomy with PCI Versus PCI Alone in Patients with STEMI Undergoing Primary PCI (TOTAL) study changed this scenario, as they showed no significant advantage for systematic on ad-hoc thrombectomy in primary PCI.38 In addition, 5-French compatible thrombus-aspiration catheters are at present available to perform selective aspiration on indication (see Figure 1).
At OLVG Amsterdam, where patent haemostasis is applied in all patients and slender TRA techniques are pursued in patients with small radial arteries, we conducted a retrospective survey of patients undergoing a second TRA procedure within 6 months after a first TRA procedure at our centre in 2015 (unpublished data). In 115 of 119 patients (96.6 %), the same radial artery was open and a second TRA was successful. In only two patients (1.6 %) RAO had occurred.
Indications for Transfemoral Approach in a Transradial High-volume Centre
e In our experience, the only absolute contraindication for the TRA and reason to switch to the TFA when performing primary PCI is if no radial artery pulsations are felt at both upper extremities. Patients in whom a left internal mammary artery graft needs to be filmed and the left radial artery is not available, or patients with dialysis shunts in the upper extremity, are also relatively contraindicated. Absence of radial artery pulsations can be the result of radial artery occlusion after previous interventions or the result of cardiogenic shock. One could argue that in cases of cardiogenic shock the TFA should be preferred to enable percutaneous mechanical cardiac support. The Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial showed in fact no reduction in mortality when intra-aortic balloon pump (IABP) was used in cardiogenic shock.39 So far, there is no evidence that other types of percutaneous mechanical cardiac support has survival benefits.40 The increased bleeding risk probably outweighs the benefit of improved haemodynamic. Based on these results, routine temporary mechanical support is not pursued at OLVG in acute patients, and IABP and left ventricular (LV) assist devices may be considered for circulatory support only in patients with refractory shock.41
When cardiac support (IABP or Impella device) is needed, our strategy is to first introduce the cardiac support device through one femoral artery and reassess the pulsatility of the radial artery; when feasible, a combined approach can be performed (i.e. TRA primary PCI plus femoral cardiac support device). The TFA is also our bail-out approach in those few cases where the TRA is not successful either because of puncture or navigation failures, or lack of support.
Conclusion
Results from our institution and from the literature show that systematically pursuing the TRA for primary PCI results in high procedural success rates and favourable clinical outcomes.18 As also demonstrated by previous studies, this is due to a lower risk of bleeding and lower mortality risk.7,8 The TRA has also the additional advantage of faster patient turn-over and enhanced patient comfort. The TRA is technically challenging and requires a longer learning curve. Moreover, the issue of RAO should not be neglected but rather prevented by patent haemostasis and miniaturisation of catheters (Slender PCI). It is fundamental that both starting and experienced TRA operators will systematically adopt the TRA during elective PCI to optimise their skills, which will eventually lead to comparable door-to-balloon times and procedural outcomes during primary PCI, but with better patient outcomes. Proficiency in the TFA will have to be maintained in cases of bail-out and special circumstances (shock primary PCI).