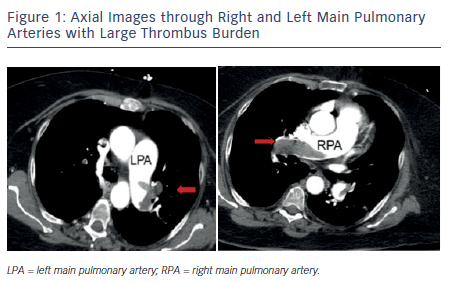
Pulmonary embolism (PE) is a common and serious manifestation of venous thromboembolism (VTE) and is an important cause of morbidity and mortality in the US. The incidence is estimated to be 50 per 100,000 but increases to 500 per 100,000 in the elderly.1 There is a wide spectrum of clinical severity with mortality estimates of 1–2 % in stable individuals and up to 30 % in patients presenting with hemodynamic instability.1 Massive (or high-risk) PE is a term used to designate patients with sustained hypotension (systolic blood pressure <90 mmHg for at least 15 minutes or requiring inotropic support, not due to a cause other than PE), pulselessness, or persistent profound bradycardia. Submassive (or intermediate-risk) PE refers to those patients with acute PE without systemic hypotension but with evidence of either right ventricle (RV) dysfunction or myocardial necrosis. RV dysfunction is characterized by RV dilation, hypokinesis, or elevation of brain natriuretic peptide (BNP); myocardial necrosis is suggested by elevated troponin. Careful clinical assessment must include appropriate risk stratification since this will influence both diagnostic and therapeutic decision-making.
Clinical Presentation
The clinical spectrum ranges from asymptomatic individuals to those presenting with syncope, shock, or sudden death. Symptom onset is typically rapid (minutes to hours) but can develop over days or weeks. The most common presenting symptom is dyspnea, followed by symptoms of pulmonary infarction, including pleuritic pain, cough, and, less commonly, hemoptysis. Symptoms of deep vein thrombosis (DVT), including leg swelling or pain, may be present. Many patients have only mild or nonspecific symptoms and asymptomatic PE is sometimes seen on computed tomography (CT) scanning. The most common signs of PE are tachypnea and tachycardia. Many patients appear anxious. Lung exam is often normal but may reveal rales or decreased breath sounds. An extremity exam may reveal a palpable cord, unilateral or asymmetric edema, tenderness, warmth, erythema, or superficial venous dilation. With more extensive clot burden, findings include hypotension, hypoxemia, altered mental status, and signs of RV strain (distended neck veins, tricuspid regurgitation murmur, accentuated pulmonic component of the second heart sound, right-sided third heart sound, parasternal lift). An example case is provided in Box 1.
Risk Stratification of Massive and Submassive Pulmonary Embolism
Early risk stratification in patients with PE is vital to guide acute management. The presence of hypotension and shock is associated with high mortality, and warrants immediate intervention with aggressive therapy. Nevertheless, select patients with PE in absence of hemodynamic instability may also have unfavorable outcomes, and benefit from more aggressive therapy. A combination of clinical features, diagnostic studies, and biomarkers are used to risk stratify these patients.
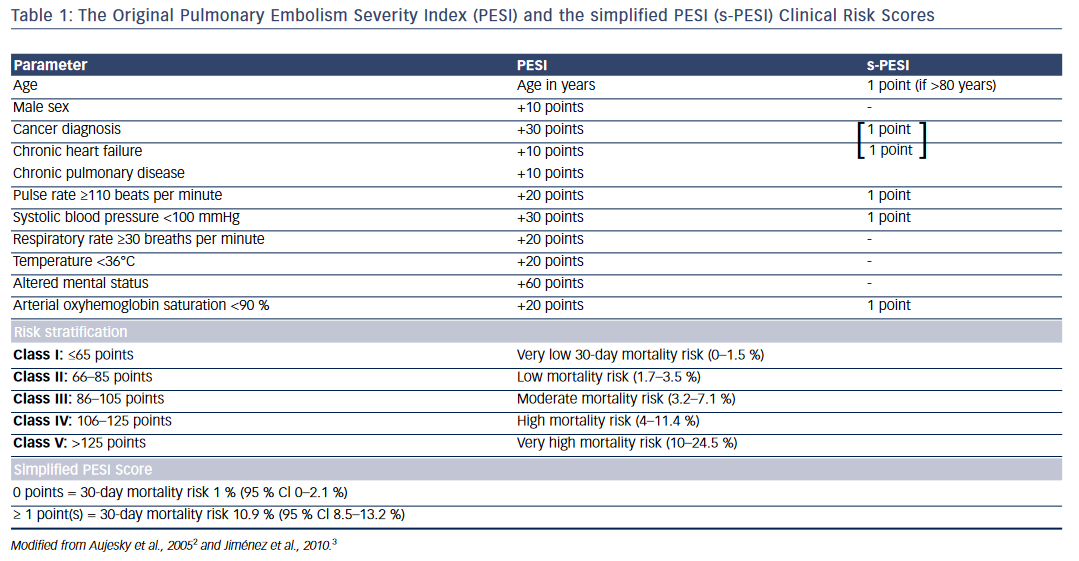
Clinical Risk Scores
One of the widely used risk scores for prognostic stratification of acute PE is the PE severity index (PESI) or its simplified version (sPESI)2,3 (see Table 1). The original PE severity index identifies 11 predictors of adverse outcome that include age >80 years, male sex, cancer, chronic heart failure, chronic obstructive pulmonary disease (COPD), tachycardia, hypotension, tachypnea, hypothermia, altered mental status, and arterial hypoxemia. The modified version—the simplified PESI index— uses a 6-point scoring system for predicting adverse outcomes: 1 point each for age >80 years, heart failure or COPD, underlying malignancy, tachycardia, hypotension, and arterial hypoxemia. Patients with PESI score >85 points (Class III–V) and sPESI ≥1 point are considered to have higher risk for adverse outcomes and mortality (see Table 1).2,3
Assessment of Right Ventricular Function
RV dysfunction as assessed by echocardiography is an important adverse prognostic marker in patients with PE.4,5 The most commonly used criteria for RV dysfunction on echocardiography include: right ventricular dilatation with RV end-diastolic diameter >30 mm, RV to left ventricle (LV) end diastolic ratio ≥0.9 and RV hypokinesia. Other criteria include paradoxical septal wall motion, pulmonary hypertension, and severe tricuspid regurgitation.4,6 RV dysfunction detected by echocardiography is associated with an elevated short-term mortality in patients with PE.7,8
Similarly, a right to left ventricular dimensional ratio of ≥0.9 on multidetector CT (MDCT) has been shown to have a 92 % sensitivity for detection of RV dysfunction. Right ventricular dysfunction by MDCT was shown to be an independent predictor for in-hospital death or clinical deterioration in patients with PE (hazard ratio [HR] 3.5, 95 % confidence interval [CI] 1.6–7.7; p=0.002), including hemodynamically stable patients.9
Cardiac Biomarkers
Cardiac troponins when elevated in patients with acute PE is suggestive of RV myocardial injury and has been shown to predict short-term mortality and adverse outcomes.10,11 In a meta-analysis of 20 studies, Becattini et al. found that elevated troponin levels were significantly associated with short-term mortality (odds ratio [OR] 5.24; 95 % CI [3.28–8.38]), with death resulting from PE (OR 9.44; 95 % CI [4.14–21.49]), and with adverse outcome events (OR 7.03; 95 % CI [2.42–20.43]). Elevated troponins also predicted high mortality in a subgroup of hemodynamically stable patients (OR 5.90; 95 % CI [2.68–12.95]). Results were consistent for both troponin I or T.12
Elevated BNP and N-terminal (NT) pro-BNP, which are reflective of RV pressure overload, have also been shown to be independent predictors of death and adverse outcomes in patients with PE.7 In a systematic review by Klok et al., patients with elevated BNP or NT pro-BNP had a 10 % risk for early death (95 % CI [8.0–13]) and a 23 % (95 % CI [20–26]) risk for an adverse clinical outcome.13
Treatment Modalities
Anticoagulation
Prompt initiation of therapeutic anticoagulation is indicated for all patients with PE unless there is a strong contraindication (e.g., active or recent severe bleeding, major surgery, or trauma). Consideration should be given to starting empiric therapy if there will be a delay in diagnosis and clinical suspicion for PE is high (e.g., using a validated prediction rule such as the Wells criteria). The immediate objective of anticoagulation is to prevent clinical deterioration, recurrent PE, and death. Rapid-acting anticoagulants include parenteral drugs (unfractionated heparin [UFH], low molecular weight heparin [LMWH], fondaparinux) as well as direct oral anticoagulants (DOACs). Compared with UFH, LMWH produces a more predictable anticoagulant effect and is the preferred drug in stable, lower-risk patients (although LMWH and fondaparinux should be avoided in patients with creatinine clearance (CrCl) <30 ml per minute or extremes of body weight; anti-Xa monitoring is an option in such cases.14–16 UFH has the advantage of a short half-life so is preferred in unstable patients who may require an intervention or thrombolysis or are at high risk for bleeding. Rapid-acting parenteral drugs should be continued for at least 5 days and overlapped with oral therapy while initiating a vitamin K antagonist, such as warfarin. Two DOACs (rivaroxaban, apixaban) were studied and approved for use as monotherapy (i.e., without initial LMWH or UFH) while two others (dabigatran, edoxaban) require at least 5 days of LMWH or UFH prior to initiation.17–22 Patients with hemodynamic instability or requiring a vena cava filter or fibrinolytic therapy were excluded from DOAC trials so the role of these agents in this setting has yet to be defined. Due to superior efficacy, continued LMWH monotherapy without transitioning to warfarin is preferred in patients with malignancy.23–25 If heparin-induced thrombocytopenia (HIT) is suspected or confirmed, fondaparinux or a direct thrombin inhibitor, such as argatroban, should be used.26,27
Systemic Thrombolysis
Use of systemic thrombolysis has been shown to decrease mortality and recurrent PE in high-risk patients who present with hemodynamic instability.28 In addition to rapid resolution of major pulmonary emboli, thrombolytic therapy is also known to decrease pulmonary artery pressure and improve RV function with a concomitant increase in LV output compared with anticoagulation with heparin alone.28,29 Thrombolytics approved for PE by the US Food and Drug Administration (FDA) include streptokinase, urokinase, and alteplase. Studies show similar efficacy for tenecteplase and reteplase, though they are not yet approved by the FDA for PE.30,31 The hemodynamic benefits of thrombolysis are seen in the first few days of treatment.28 Clinical and echocardiographic data indicate that >90 % of patients with PE respond to thrombolytics within the first 36 hours.32
There are conflicting data for the use of thrombolytic therapy in hemodynamically stable patients with acute PE. Konstantinides et al.33 reported that in patients with acute PE without hypotension, but with RV dysfunction, alteplase reduced the primary end point of in-hospital death or clinical deterioration requiring escalation of treatment, with no significant elevation in major hemorrhagic complications. However, the effect of thrombolysis on mortality in these intermediate-risk patients is not clear. In a meta-analysis of randomized controlled trials of thrombolytic therapy, Marti et al.34 found that thrombolytic therapy was associated with a significant reduction in overall mortality (OR 0.59; 95 % CI [0.36–0.96]). While this reduction in early mortality was not statistically significant after exclusion of studies with high-risk PE, thrombolytic therapy did significantly reduce the incidence of PE mortality, death or treatment escalation, and PE recurrence. Further, there was no significant difference between alteplase, tenecteplase, or older thrombolytics. Similar results were found in another meta-analysis by Chatterjee et al.35 though in their analysis, there was a significant reduction in mortality even among patients with intermediate-risk PE.
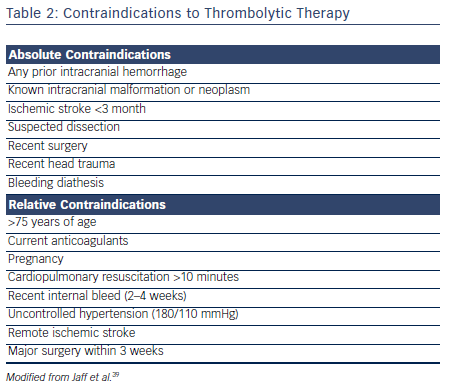
Thrombolytic therapy is also associated with increased incidence of major bleeding.34–36 The Pulmonary Embolism Thrombolysis (PEITHO) investigators found that single-dose tenecteplase poses a high risk for hemorrhagic stroke when used in hemodynamically stable patients with acute PE.30 Similarly, major hemorrhage (OR 2.91; 95 % CI [1.95–4.36]) and fatal or intracranial bleeding (OR 3.18; 95 % CI [1.25–8.11]) were more likely among patients receiving thrombolysis in the meta-analysis by Marti et al.34 The Moderate Pulmonary Embolism Treated with Thrombolysis (MOPETT) trial used half the conventional dose of tissue plasminogen activator (tPA) in the treatment of intermediate PE.37 At a reduced dose, tPA was shown to significantly reduce the pulmonary artery systolic pressure without any significant increase in major bleeding.38 Careful assessment for bleeding risk is warranted in all patients being considered for thrombolytic therapy (see Table 2).
The current guidelines recommend systemic thrombolysis in patients with high-risk PE with hemodynamic compromise in the absence of contraindications. However, guidelines do not recommend routine use of systemic thrombolysis in all patients with intermediate-risk PE. A careful risk/benefit analysis should be performed on an individual basis in patients with intermediate-risk PE.
Percutaneous Catheter-directed Treatment
Percutaneous catheter-directed therapy (CDT) involves the removal or disruption of obstructing thrombi from the main pulmonary arteries and/ or local administration of small dose of thrombolytics directly into the pulmonary artery. The various interventional options include thrombus fragmentation with pigtail or balloon catheter, rheolytic thrombectomy, suction thrombectomy with aspiration catheters, and rotational thrombectomy.39 Concurrent administration of local thrombolytics can be performed via multiple side-hole catheter(s) in the pulmonary artery with or without the application of ultrasound energy as used in the EKOS device (EkoSonic Endovascular). Catheter-based therapy has the advantage of being better tolerated in patients with tenuous hemodynamics compared with surgical embolectomy and requiring lower doses of thrombolytic compared with systemic therapy. However, CDT is only effective in main pulmonary artery or its major branches and with less organized thrombus. The efficacy of CDT for thrombus removal is limited by relatively small size of the aspiration catheters compared with the size of the pulmonary artery.
There are limited good quality data on hard outcomes using CDT in patients with acute PE. In a systematic review on CDT in acute PE, Kuo et al.40 included 594 subjects from 35 nonrandomized studies and showed 87 % clinical success with CDT in terms of stabilization of hemodynamic parameters, resolution of hypoxia, and survival to discharge. Pooled risk for minor and major complications was 7.9 % and 2.4 %, respectively.
In a recent single-arm multicenter study in patients with both massive (n=31) and submassive PE (n=119), Piazza et al.41 demonstrated that ultrasound-facilitated, catheter-directed, low-dose fibrinolysis decreased RV size, pulmonary pressures, thrombus burden, and reduced incidence of intracranial hemorrhage. The 30-day mortality in this group was 2.7 % with 10 % incidence of major bleeding.
Nevertheless, large prospective studies are lacking, and the ideal CDT protocol, particularly for submassive PE, remains unclear. There are limited data comparing the efficacy of systemic thrombolysis and CDT for management of acute PE. CDT should be considered in high-risk patients as an alternative to surgical embolectomy when systemic thrombolysis is contraindicated or has failed or in intermediate–high-risk patients if the anticipated risk for bleeding with systemic thrombolytic therapy is high.42
Surgical Embolectomy
Surgical embolectomy is an open surgical procedure in which clots are removed from the right atrium or ventricle or main/proximal pulmonary arteries. It is indicated in patients with massive PE (and possibly in select patients with submassive PE) who have a contraindication to thrombolysis or when thrombolysis or catheter-based mechanical clot disruption has failed. A wide range of mortality rates have been reported (6–46 %).43,44 In a recent report describing 105 patients who underwent surgical embolectomy (49 hemodynamically unstable; 56 hemodynamically stable), overall operative mortality was 6.6 % (10.2 % for unstable patients; 3.6 % for stable patients).45 Of 11 patients requiring preoperative cardiopulmonary resuscitation, four died. Six-month, 1-year, and 3-year survival rates were 75 %, 68.4 %, and 65.8 % for unstable PE, and 92.6 %, 86.7 %, and 80.4 % for stable PE, respectively. These findings suggest that surgical embolectomy is an important option that can provide reasonably good outcomes if significant experience is locally available and patients are carefully selected.
Vena Cava Filters
In patients with acute PE who cannot safely receive anticoagulation, placement of an inferior vena cava (IVC) filter is indicated (even in the absence of lower extremity clot). Filters are placed via catheter in the infrarenal portion of the IVC; in certain circumstances, filters may be placed in other locations (superior vena cava if upper extremity clot is thought to be the source of PE; suprarenal IVC for renal vein clot). Observational studies suggest IVC filters may reduce PE-related mortality in the acute setting but these studies have methodologic limitations.46,47 Eight-year follow-up of a randomized study in 400 patients with DVT (with or without PE), all of whom had initial anticoagulation for at least 3 months, showed patients who received a permanent IVC filter had a reduced risk for recurrent PE (6.2 % versus 15.1 %; p=0.008), but an increased risk for recurrent DVT (35.7 % versus 27.5 %; p=0.042), and no overall effect on survival.48 A more recent study of patients with acute PE and additional risk factors for recurrence compared retrievable IVC filters plus anticoagulation to anticoagulation alone.49 Filter retrieval was accomplished in the majority of patients at 3 months and anticoagulation was continued for at least 6 months. At 6 months, recurrent PE was seen in 3.5 % of patients in the filter group and 2.0 % of patients in the control group (RR with filter, 1.75; 95 % CI [0.52–5.88]; p=0.54). There is therefore no clearly established role for IVC filters in patients with PE who can receive anticoagulation. After filter placement, patients should be periodically reassessed for initiation of anticoagulation and filters should be removed once the risk for recurrent VTE is felt to be acceptably low, usually within several months (sometimes up to a year). Filter placement is associated with a variety of complications, including malpositioning of the filter, guide-wire entrapment, and insertion site thrombosis, hematoma, or arterial-venous fistula. Long-term complications include chronic thrombosis/occlusion of the IVC, which has been reported in 3–30 % of patients, as well as IVC perforation, filter fracture, and migration.
Approach to Patient
Patients with Acute PE and Shock or Hypotension
High Risk
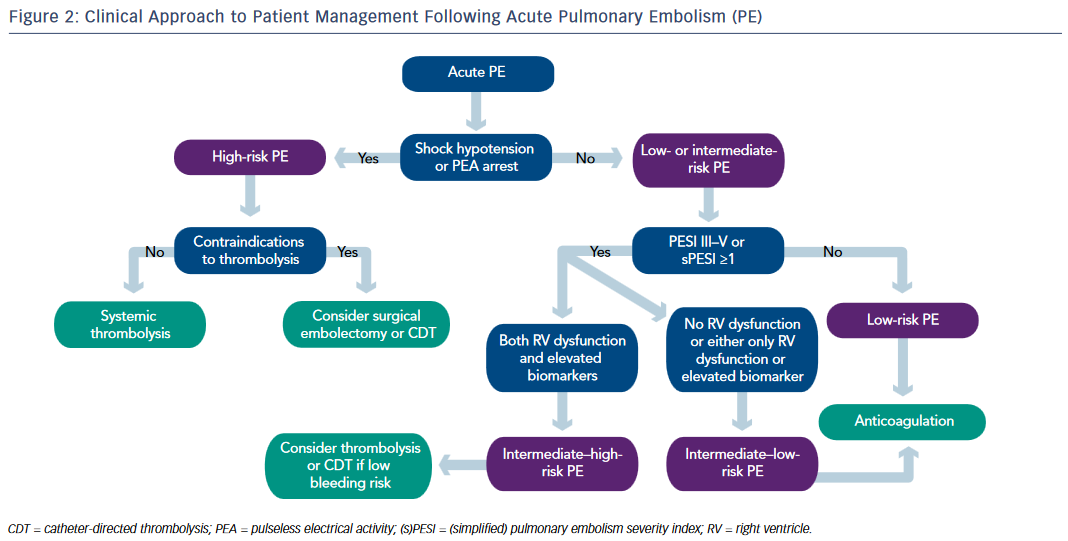
In addition to providing hemodynamic and respiratory support, high-risk unstable patients with suspected PE should be started on intravenous UFH. Early thrombolytic therapy is strongly recommended in these patients in the absence of contraindications. Surgical embolectomy is recommended for patients in whom thrombolysis is either contraindicated or unsuccessful. At experienced centers, percutaneous CDT can be considered as an alternative to surgical pulmonary embolectomy (see Figure 2).
Intermediate Risk
Parenteral anticoagulation with UFH or LMWH or fondaparinux is recommended without delay in patients with intermediate–high risk PE. Echocardiogram and cardiac biomarkers should be obtained. If there is evidence of RV dysfunction and elevated cardiac biomarkers, the patient is deemed as intermediate–high risk. Systemic thrombolysis or catheterdirected thrombolysis can be considered in selected intermediate– high risk patients who are at low risk for bleeding. Patients with RV dysfunction with normal biomarkers or elevated biomarkers with normal RV function are deemed as intermediate–low risk and treatment with anticoagulation alone may suffice (see Figure 2).
Routine use of IVC filters in patients with PE is not recommended. IVC filter should be considered if there is an absolute contraindication to anticoagulation or recurrent PE despite adequate anticoagulation.
Pulmonary Embolism Response Team
In order to provide individualized approach to patients with intermediateand high-risk PE, medical centers should develop a multidisciplinary team and uniform institutional protocols for management of these patients.50 In their initial experience at Massachusetts General Hospital the Pulmonary Embolism Response Team (PERT) was activated in about 400 patients over the initial 2.5 years, with 60 % of activations from emergency department and 20 % from intensive care units. While it is too early to estimate the impact of PERT on patient outcomes, it can streamline care with rapid access to multiple specialties and has the potential to provide a uniform approach to patients with massive and submassive PE.51
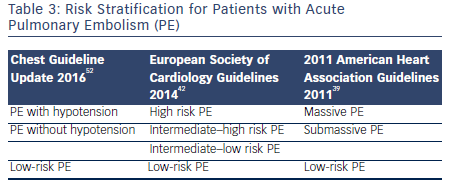
Guidelines for Management of Acute PE
There are three guideline documents that address the diagnosis and management options for patients with acute PE. The 2014 European Society of Cardiology guidelines,42 the 2011 American Heart Association guidelines for massive and submassive PE,39 and the 2016 update of the Chest guidelines for venous thromboembolic disease.52 The guidelines use slightly different terminology in risk stratification for PE. This is summarized in Table 3. Broadly, the guidelines are consistent in recommending systemic fibrinolysis in patients with massive PE and considering CDT or surgical embolectomy as alternatives for patients with contraindications to systemic thrombolysis at centers with local expertise. The Chest guidelines in general are more conservative and recommend against thrombolysis in patients with PE without hypotension unless the patients deteriorate after starting anticoagulation. The guidelines also consistently recommend against the routine use of IVC filters.