Heart failure (HF) and chronic obstructive pulmonary disease (COPD) comorbidity poses substantial diagnostic and therapeutic challenges in acute care settings. Outcomes of this comorbidity are worse than in either disease alone.1,2 A hospital diagnosis of COPD is an independent predictor of all-cause and non-cardiovascular mortality in HF patients,3–5 associated with decrease in use of evidence-based HF medications and longer hospitalisation durations.6 Prevalence of co-existent COPD diagnosis in hospitalised HF patients is summarised in Table 1.5–16 Half of the patients with an acute exacerbation of COPD are reported to have echocardiographic evidence of left ventricular failure.1,2
Pathophysiology of Cardiopulmonary Continuum in Acute Exacerbations
Evidence increasingly suggests that both HF and COPD can be interpreted as systemic disorders associated with low-grade inflammation, endothelial dysfunction, vascular remodelling and skeletal muscle atrophy.5,17,18
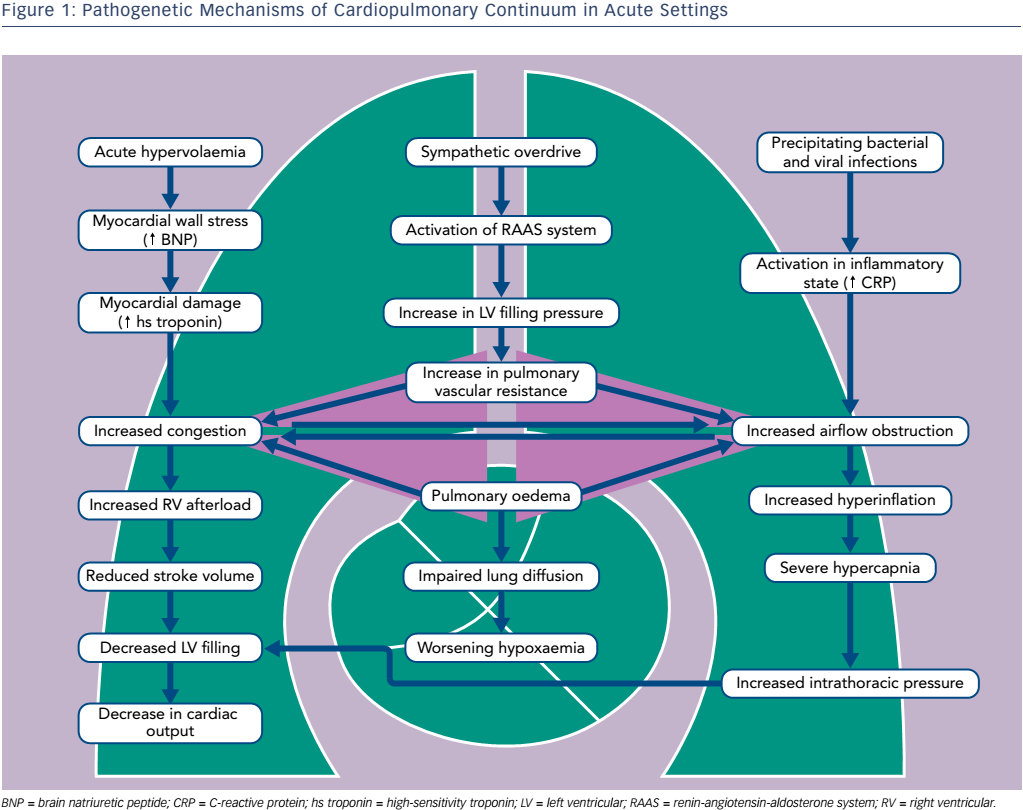
Abrupt haemodynamic, ventilatory and fluid content changes superimpose on chronic structural and functional abnormalities caused by long-term co-existence of cardiac and pulmonary conditions. Patients with COPD and HF have a combined obstructive and restrictive type of pulmonary dysfunction.19 COPD is characterised by obstructed airflow, destruction of pulmonary tissue in emphysema and respiratory muscle weakness. In turn, progressive heart enlargement taking thoracic space, venous congestion, interstitial fibrosis, pleural effusions and substantial atelectasis all contribute to pulmonary compression in HF. Typically for COPD, decrease in Oxygen (O2) arterial pressure and an increase in carbon dioxide (CO2) arterial pressure in case of coincident HF is combined with alteration of lung diffusion capacity due to the thickening of the alveolar septa, reduction in alveolar–capillary membrane conductance and lung remodelling with collagen deposition.17–19
Acute pulmonary oedema typically causes the dynamic airflow obstruction due to interstitial fluid and bronchial mucosal swelling (see Figure 1).20–22 In 19 % of patients hospitalised for congestive systolic HF, initial airway obstruction was found but had disappeared in 47 % of these patients after re-compensation. Bacterial and viral infections as well as inflammatory process in the small airways are important precipitating factors.23 Progressive respiratory failure usually increases airway obstruction, hypoxaemia and ventilation–perfusion mismatch.
In acute phases of both entities, elevated biomarkers of neurohumoral activation, myocardial damage and inflammation have been found.4 Severe hypoxaemia, cardiac stress, increased sympathetic nervous and platelet activation may contribute to myocardial necrosis. Of note, undiagnosed subendocardial infarctions are revealed in autopsies of patients who have died during acute exacerbation of COPD.24 Importantly, the substantial elevation of natriuretic peptides was reported even when the COPD patient had no clinical or echo signs of overt right ventricular failure, with the subsequent fall of concentration during the first days of treatment in parallel with the decrease in pulmonary arterial pressures.
The CardioMEMS Heart Sensor Allows Monitoring of Pressures to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) study analysis proved the importance of pulmonary vascular resistance and increased pulmonary artery pressure for decompensation of both diseases.16 Pulmonary vascular disease associated with hypoxic vasoconstriction was shown to be an important risk factor for respiratory exacerbations and mortality in patients with COPD. It is believed that products of tobacco smoke induce inflammatory changes and further pulmonary vasculature remodelling.
The true prevalence of pulmonary hypertension among COPD patients is not known, and genetic predispositions may have a role in different susceptibility of COPD patients towards pulmonary hypertension.17,23
Diagnostic Challenges of Dyspnoea in Patients with Heart Failure and Chronic Obstructive Pulmonary Disease
Only 37 % of patients with a history of pulmonary disease were correctly identified as presenting with HF by the emergency physicians.25
Wheezing may be audible in HF patients with acute congestion, while crackles of pulmonary oedema are frequently not heard in a hyperinflated chest.26 The radiographic appearance of pulmonary oedema may be atypical in patients with emphysema because of the destruction of the pulmonary vascular bed or additional shadows. Vascular redistribution may be due to COPD rather than raised left atrial pressure. When differential diagnosis includes parenchymal lung disease, a computed tomography (CT) scan of the chest could be useful. Characteristic findings include ground-glass opacities, pleural effusions and cardiomegaly. However, the cardiothoracic ratio may remain normal if the heart tends to become long and narrow in a hyperinflated chest.
Echocardiography also has limitations in the differentiation between acute HF and COPD. The estimated prevalence of unsatisfactory ultrasound image quality reaches up to 50 % in severe airflow obstruction.27 High pulmonary hypertension is diagnosed in almost one-fifth of HF patients irrespective of left ventricular ejection fraction. Due to elevation in leftsided filling pressures, 52.5 % patients with HF with preserved ejection fraction have been diagnosed with pulmonary hypertension.22,23
Lung ultrasonography is recommended as a useful tool to identify and monitor congestion in acute care.28–30 Simultaneously, it helps visualise pleural effusion, pneumothorax or lung consolidation. When the fluid leaks into the interstitial space the air–fluid interface creates the acoustic substrate for B-lines.
Non-invasive indices of right ventricular size and function may add incremental prognostic value in patients with acute dyspnoea.31 B-type natriuretic peptide (BNP) plasma levels serve as an early sensitive indicator of right ventricular (RV) dysfunction.25 Values >500 pg/ml are highly suggestive of overt congestive heart failure (CHF). Values between 100 and 500 pg/ml should alert to the possible presence of HF complicating COPD.32 A high negative predictive value of concentration <100 pg/ml is preserved in cohorts of patients with a dual diagnosis.
Therapeutic Dilemmas in Comorbid Cardiopulmonary Disorder
No large prospective studies have specifically examined the impact of beta2-agonists on HF outcomes, as well as safety and effectiveness of beta-blockers for patients with co-existent HF and COPD. Management of these patients is based mainly on clinical expertise and observational data, which currently are reassuring for concomitant use of beta2- agonists and beta-blockers in a comorbid cardiopulmonary condition. Information about the treatment of this patient population in acute settings is particularly limited.
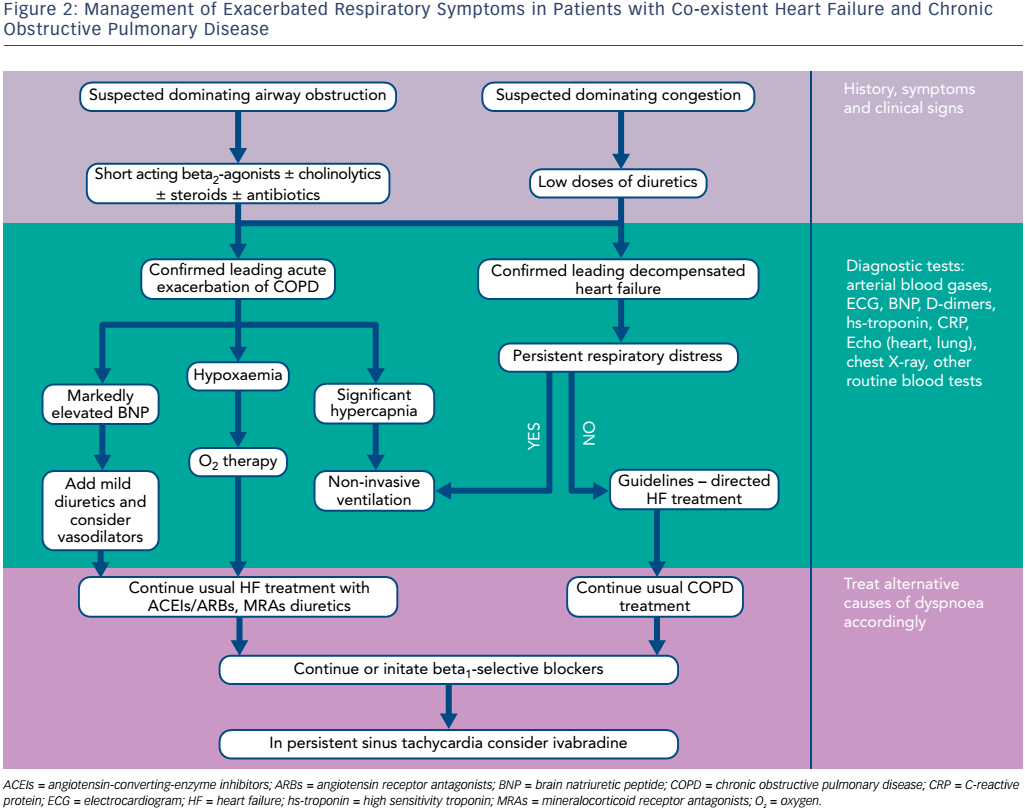
Suggested management pathways of concurrent HF and COPD are presented in Figure 2.
Regular Respiratory Treatment in Acute Heart Failure
Surprisingly, many acute decompensated HF patients receive inhaled bronchodilators even without a history of COPD.13,33
Data from Premier Perspective® database showed that among 164,494 HF hospitalisations, 53 % received acute respiratory therapies during the first two hospital days: 37 % received short-acting inhaled bronchodilators, 33 % received antibiotics and 10 % received highdose corticosteroids.13 Acute respiratory therapy was associated with higher odds of in-hospital mortality, admissions to an intensive care unit, late intubation, and was more frequent among the 60,690 hospitalisations with chronic lung disease. Such co-treatment may be explained by complexity in differential diagnosis of cause of acute dyspnoea in typical practice. Rates of initial co-treatment were above 50 % even among patients who underwent an early diagnostic testing with natriuretic peptides or chest radiographs. Therefore, HF is regularly treated as a broader cardiopulmonary syndrome, with less than half of patients treated exclusively for HF. Bronchial mucosal swelling, peribronchial oedema, bronchoconstriction and alveolar fluid accumulation may lead to a reversible airway obstruction in singular acute HF; however, whether bronchodilators improve symptoms of dyspnoea in this case is unknown.
Use of Beta2-agonists and Cardiovascular Outcomes
Beta-agonists were reported to significantly increase tachycardia in patients with obstructive airway disease, which in turn may increase myocardial oxygen consumption and electrical instability; these effects are specifically detrimental in failing myocardium. Several retrospective analyses raised concerns about the higher risk of arrhythmias, acute ischaemic events, HF hospitalisations and mortality in patients using beta2-agonists.34–36 However, these data were mostly collected two decades ago, when beta-blockers were roughly used by 30 % of HF patients, and overall treatment for HF and ischaemic heart disease was substantially different. Later studies demonstrated a strong protective effect of cardiac agents against bronchodilator associated risks.37–40 A recent multicentre study (Towards a Revolution in COPD Health [TORCH]) with more than 6,000 patients with COPD (41 % of them taking cardiovascular medications) showed no increase in overall and cardiovascular-related adverse events in the salmeterol group.38–39 Likewise, adjustment to detailed clinical information and levels of natriuretic peptide in a longitudinal cohort study of HF patients eliminated differences in mortality between beta2-agonist users and non-users, thus suggesting that bronchodilator use may be a marker of a more severe disease.40
Nevertheless, in view of the absence of strong evidence or accepted recommendations, bronchodilators should be used with caution in acute settings with patients with underlying HF, especially in those having tachyarrhythmias. Given the previously reported dosedependent increase of risk of adverse cardiovascular outcomes in observational studies, reduction of dose and frequency of beta2- agonists or temporary withdrawal until haemodynamic stabilisation may be considered, until safety data are available.36,37
Beta-blockers Improve Outcomes in Respiratory Decompensation
To date, extensive observational data have been accumulated of protective effects of beta-blockers on mortality and exacerbations in patients with COPD.41–49 Two studies were performed in acute settings.50,51 A single-centre analysis found that beta-blocker use was an independent predictor of survival to hospital discharge, with no evidence that these agents reduce the beneficial effects of shortacting beta2-agonists in collateral use.51 In a cohort of patients with cardiovascular disease admitted due to acute COPD exacerbation to 404 acute care hospitals, there was no association between betablocker therapy and in-hospital mortality, 30-day readmission or late mechanical ventilation.50 Of note, receipt of non-selective betablockers was associated with an increased risk of 30-day readmission compared with beta1-selective blockers.
In a meta-analysis of 15 retrospective studies of 21,596 patients with COPD, the pooled estimate for reduction in overall mortality attributed to the use of beta-blockers was 28 % (95 % confidence interval [CI], 17–37 %) and for exacerbations was 38 % (95 % CI, 18–58 %). The reduction in mortality was 26 % (95 % CI, 7–42 %) in the subgroup with known HF.52 However, no results from randomised controlled trials are available to date.
Despite evidence-based indications, numerous reports reveal that most COPD patients with concurrent cardiovascular disease are denied the protective effect of beta-blockers. Underuse of beta-blockers stems from the concern regarding beta-2 receptor antagonism and associated bronchoconstriction. For example, among patients with COPD admitted to hospital for acute HF in a large Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF) registry, betablockers were underutilised at discharge.14 Recent data suggest that the prescription of beta-blockers in patients with heart disease has doubled in the last decade in both patients with and without COPD.41
A number of studies indicate that cardioselective beta-blockers exert minimal impact on reversible or severe airflow obstruction. A cochrane review including 20 randomised trials of cardio-selective beta-blockers in COPD found no significant effect on forced expiratory volume in 1 second (FEV1) or bronchodilator response after a single dose or up to 12 weeks of treatment.42 In three small randomised controlled trials examining beta-blockers in patients with HF and concurrent COPD,43–45 cardioselective beta-blockade was well-tolerated and beneficial effects on lung function were seen.
Besides clear cardioprotective action, beta-blockers may be beneficial due to modulation of the immune response and improved clearance of bacteria from the circulation during systemic infections. All these data together advocate continuation or initiation of beta-blockers (preferably beta1-selective) during acute respiratory exacerbation in patients having concurrent HF and COPD.
The Interference of beta-blockers and beta-agonists
Data on drug interaction between beta-blockers and bronchodilators are scarse. In a retrospective cohort study of acute exacerbation of COPD, no evidence that beta-blockers reduce the beneficial effects of short-acting beta-agonists when the two are used in combination was found.51 Contrary, it has been suggested that beta-blockers may be beneficial by enhancing sensitivity to endogenous or exogenous betaadrenergic stimulation and improve bronchodilator responsiveness by upregulation of beta-receptors within the lung.41,42 Moreover, beta-blockers may blunt the potential cardiac toxicity of short-acting beta-agonists. The aim to preserve bronchodilator action of beta2- agonists grounds the choice of selective beta1-blockers in acute cardiorespiratory decompensation. In acute COPD, normal doses of selective beta1-blockers appear to be safe and well tolerated.
Role of Diuretics and Vasodilators in Co-existent Heart Failure and Chronic Obstructive Pulmonary Disease
Treatment of acute HF in COPD patients with diuretics improves gas exchange by removal of lung water, improvement of lung compliance and increase in FEV1.53,54 Impressive reduction of respiratory hospitalisation rates in the COPD cohort in the CHAMPION trial was driven by changes in diuretic therapies in response to elevated pulmonary artery pressure data.16 A BNP level of >500 pg/ml indicates that HF therapy should be initiated or upgraded in addition to COPD treatment.55 Intriguing data are published suggesting that BNP is a bronchorelaxant and a potential new drug for COPD.56 Early administration of diuretics and vasodilators may improve outcomes of patients with acute exacerbation of comorbid HF and COPD.
Effects of Renin-angiotensin-aldosterone System Blockers and Ivabradine in Chronic Obstructive Pulmonary Disease
In patients with HF and co-existent COPD, angiotensin-convertingenzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) carry an additional benefit by decreasing levels of angiotensin-II, which is a potent pulmonary airway constrictor.57 Therefore, these HF medications reduce airways obstruction, decrease pulmonary inflammation and pulmonary vascular constriction, and improve the alveolar membrane gas exchange. Aldosterone antagonists also exhibit a positive effect on gas diffusion protecting the alveolar–capillary membrane.
The heart rate-reducing agent, ivabradine, which selectively inhibits sinoatrial funny current (If) channels, has been shown to similarly reduce cardiovascular risk in both COPD and non-COPD patients, thus presenting an effective alternative measure to reduce sinus tachycardia in case of a complicated comorbid decompensation.57
Non-invasive Ventilation
Non-invasive ventilation (NIV) improves the outcomes of patients with acute respiratory failure due to hypercapnic exacerbation of COPD or HF with acute pulmonary oedema. NIV improves gas exchange, accelerates the remission of symptoms, reducing the need for endotracheal intubation, hospital mortality and hospital stay when compared with conventional O2 therapy.30,31 In patients with cor pulmonale secondary to a chronic pulmonary disease like COPD, the use of biphasic positive airway pressure can improve the right ventricular function and decrease plasma levels of natriuretic peptides.
Currently there is no direct evidence for the treatment of concomitant HF or COPD that is different from the accepted clinical guidelines for both diseases.57,58
The specific role of pulmonary comorbidity in the treatment and outcomes of cardiovascular disease patients was not addressed in any long-term prospective study. Randomised controlled trials to elucidate effects of cardioselective beta1-blockers on pulmonary function in COPD as well as to evaluate their interaction with long-acting bronchodilators are ongoing (clinicaltrials.gov/show/NCT01656005).
The common practice of withholding beta-blockers in COPD patients seems to be unsafe, and cardioselective beta1-blockers may be preferable to non-selective until new evidence is available.