Today, the interest in optimal treatment of acute coronary syndrome (ACS) has never been greater. Several factors have contributed to this including an expanding patient population, the poorer prognosis of patients with ST-segment elevation myocardial infarction (STEMI) and multivessel disease and the recent evidence from randomised trials suggesting that multivessel percutaneous coronary intervention (PCI) is not only safe but reduces major adverse cardiac events (MACEs).1–3
In a recent publication of The Lancet, Thomas Engstrøm and his colleagues reported findings of the PRImary PCI in MULTIvessel Disease (DANAMI-3 – PRIMULTI) study, which investigated whether complete revascularisation during the index admission of STEMI patients with multivessel disease reduced the risk of future events.1 In this paper Engstrøm, the principal investigator, shares his view on this challenging patient population and the rationale and outcomes of the PRIMULTI study.
Q. Why is ACS such a challenge to interventional cardiologists?
Dr Engstrøm: The basic disease in atherosclerotic heart disease is the accumulation of lipids and calcium in the arteries that narrows the vessels and gives rise to ischaemia in the heart. In the case of ACS, on top of this, you also have a plaque rupture and thrombus formation, so you have a dual problem compared to stable angina patients.
Q. Do you see an increase in ACS patients versus stable coronary heart disease presenting at your cath lab?
Dr Engstrøm: Yes, we noted that in previous times about 30–40 % of patients were ACS patients, but they now represent more than half of our patients. Today, two out of three are ACS patients. I think that the threshold for admittance of patients with ACS is lower now so patients with very small variations in troponins are now admitted very fast for angiography. This was not the case in the past.
Q. What do the European Society of Cardiology (ESC) guidelines recommend today for the treatment of STEMI patients with multivessel disease?
Dr Engstrøm: The ESC guidelines recommend that you take care of the STEMI vessel and re-open the occluded vessel, for which the patient was admitted to the hospital. You only do additional PCI or revascularisation on multivessel stenoses if you have ongoing ischaemia, meaning you have ST-segment changes, continuous pain, cardiogenic shock, unstable arrhythmia and unstable haemodynamics.4 Otherwise, if you follow the guideline recommendations, you leave the other stenoses untreated.
Q. What drove you to conduct the PRIMULTI study?
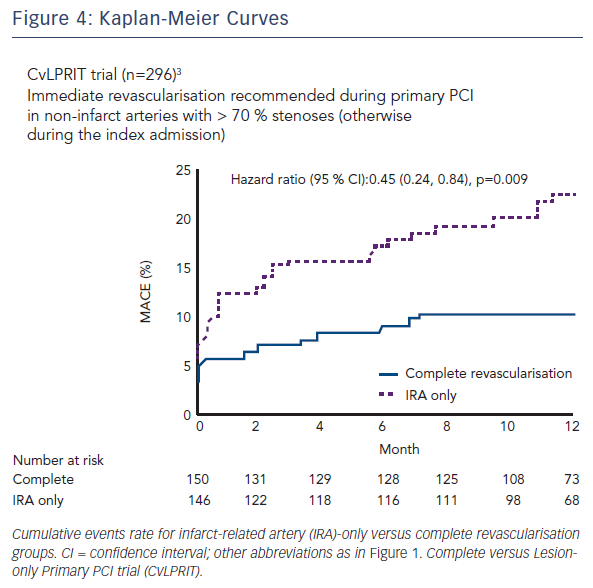
Dr Engstrøm: The main reason was the fact that physicians treat STEMI patients with multivessel disease quite intuitively. Some physicians perform full revascularisation, other physicians only treat the culprit lesion and others only consider severe or proximal stenoses for treatment. The field was very naïve until the Preventive Angioplasty in Acute Myocardial Infarction (PRAMI)2 and the Complete versus Lesion-only Primary PCI trial (CvLPRIT)3 studies came out. The PRIMULTI investigators wanted to extend the results from PRAMI and CvLPRIT into a larger scale study, simply with more patients.
Q. What methodology was used in the PRIMULTI study?
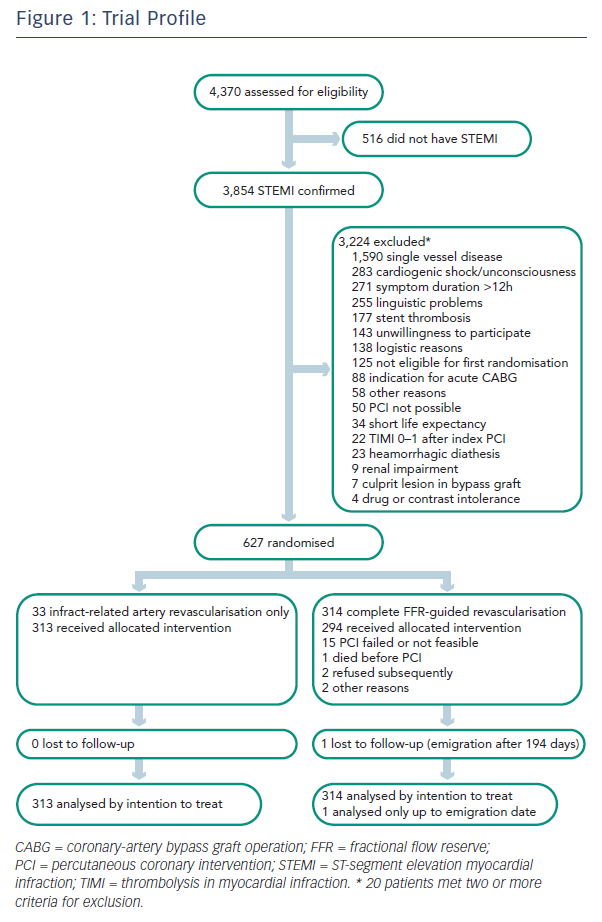
Dr Engstrøm: Patients were admitted for STEMI treatment on the basis of regular indications: symptoms for more than 12 hours and ST-segment elevation on ECG. Then, primary angioplasty was performed to relieve the thrombosis and stent the vessel.
If patients had another stenosis with at least 50 % angiographic narrowing, patients were randomised either to no further treatment other than optimal medical therapy or to fractional flow reserve (FFR)- guided PCI of the noninfarct-related lesions (see Figure 1).
FFR guidance meant that if the FFR was below 0.80, lesions were stented. If FFR was above 0.80, lesions were left untreated. If angiographically a stenosis was above 90 %, the physician was allowed to eliminate the FFR measurement and go directly to PCI treatment.
Patients were followed for at least one year. The primary endpoint was a composite of all-cause death, non-fatal myocardial infarction and the need for ischaemia-driven repeat revascularisation.
Q. What is unique about the PRIMULTI study?
Dr Engstrøm: First, the PRIMULTI study is the largest study to date on this topic. Second, we now recognise that multivessel disease is more widespread than we had originally thought. It is an interesting topic; it is a big study, carried out within a contemporary framework with regard to antithrombotic therapy, second-generation drug-eluting stents, etc.
Q. How is the PRIMULTI study different from previously published studies such as the PRAMI2 and CvLPRIT3 trials?
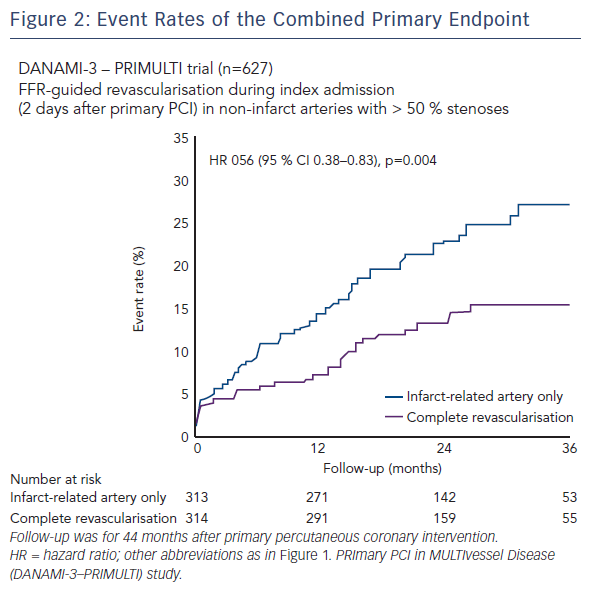
Dr Engstrøm: The results from the three trials are quite uniform (see Figures 2, 3 and 4). In all three trials there is between 45 % and 65 % relative reduction in the primary endpoints. The outcomes all point in the same direction. However, there are some differences between the three studies:
- First, the effect on hard endpoints was quite striking in PRAMI. In the PRIMULTI trial, we could not find any effect on hard endpoints, such as death or non-fatal myocardial infarction. We believe it might be due to selection bias in the PRAMI study, since each centre only recruited 19 patients per year. On the other hand, in the PRIMULTI study, we randomised 105 patients per centre per year. The PRIMULTI lesions might have been less severe than the PRAMI lesions. The CvLPRIT trial situates somewhere in between.
- Second, the primary endpoint of PRAMI was death, myocardial infarction and refractory ischaemia. In the CvLPRIT study, it was death, myocardial infarction, heart failure and ischaemia-driven revascularisation. In the PRIMULTI study, it was death, myocardial infarction and ischaemia-driven revascularisation. The differences between primary endpoints were more subtle but nevertheless, primary endpoints were different between studies.Third, in PRAMI and CvLPRIT, the decision to do PCI in the noninfarct-related arteries was based on angiographic judgement of lesion severity. In the PRIMULTI study, a more physiological assessment was preferred, with FFR measurements as part of the protocol. FFR guidance in the PRIMULTI trial changed the initial management plan in almost one third of patients, as lesions that had appeared significant on angiography were nonsignificant based on FFR.
- Finally, another difference was the strategy for noninfarctrelated artery PCI. This was done immediately in the PRAMI study, meaning at the time of the primary PCI. In the PRIMULTI study, however, PCI was staged within two days after admission for STEMI treatment.
Q. What is a realistic complete revascularisation strategy in real-world practice? What is the best timing for a complete revascularisation strategy?
Dr Engstrøm: We do not yet have sufficient data to support one strategy over another. Physicians in favour of acute full revascularisation believe you relieve all ischaemia at once, which potentially benefits the left ventricle. However, if you treat all ischaemic lesions acutely, the patient is exposed to more stress, more contrast and longer procedures. In that context, within two days after the primary PCI you have time to prepare your additional PCI, and you have less stressful operations on the patient because the treatment is done over two sessions. You also have the ability to do precise FFR measurements because the jeopardised microcirculation may influence the FFR unpredictably in the acute phase.
There are a number of unanswered questions in this regard, but we believe assessment of noninfarct-related arteries within two days is safe in STEMI patients. This is our strategy moving forward. This is applied to all patients without ongoing ischemia during the primary procedure. If there is continuous ischaemia, I believe we should immediately treat all ischaemic lesions.
Q. Does angiographic assessment of stenosis in the noninfarct-related arteries offer sufficient information to select lesions for additional treatment?
Dr Engstrøm: We don’t think so. We do think that the Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME)5 studies show clearly that FFR is superior to angiographic assessment. In the acute phase, it is even more difficult to do an angiographic assessment correctly. For example, high adrenaline levels in the blood could contract the arteries and can provide false impressions about the stenosis severity. We definitely think a physiological assessment with FFR is better.
Q. In the article published in The Lancet, you state the following, “Our results show that it is safe to do a complete revascularisation in this particular patient population. Although complete revascularisation should be recommended, any condition that makes complex PCI unattractive may support a more conservative strategy for the infarct-related artery PCI only.” Under what conditions would a more conservative approach be recommended?
Dr Engstrøm: Our study clearly showed that if you do full revascularisation, you can avoid the need for later revascularisation. However, in cases where you have a complex lesion that is only suitable for an unacceptable complex PCI, then the result and outcome of the PCI may be inferior. In those cases, you can leave these types of lesions alone since full revascularisation in our study had no effect on hard endpoints.
“ We believe assessment of noninfarct-related arteries within two days is safe in STEMI patients. This is our strategy moving forward.”
Q. Does the PRIMULTI study show that FFR further improves outcomes in STEMI when FFR is used as part of a multivessel PCI approach?
Dr Engstrøm: We may not yet be able to make that statement, but we can say that FFR-directed full revascularisations are safe, feasible and can prevent the need for further revascularisation.
Some of the patients who were randomised to FFR-guided PCI actually had no additional PCI because FFR was above 0.80 in all lesions. And of course, these patients do not face the risk for stent thrombosis in those lesions. In this context, I think you can say FFR further improved outcomes.
Q. How confident are you that measuring FFR in STEMI patients is safe?
Dr Engstrøm: I am quite confident FFR is safe if it is done after some hours or one to two days after the primary PCI. A recent publication from Ahmed et al.6 describes the largest study to date showing FFR is feasible and safe in STEMI and NSTEMI patients. As the study included both academic and regional hospitals, without a track record in coronary physiology, the observations are representative of real-world clinical practice.
Q. There are concerns that FFR may not be reliable in STEMI patients. Is that a valid concern?
Dr Engstrøm: It is a valid concern, but it is only relevant in the acute phase if the microcirculation is jeopardised. In that case, you are not able to create sufficient hyperaemia and get the true FFR. There are data and papers that suggest the concern is a bit overrated. However, as it cannot be fully excluded, we believe it is not a problem to postpone the FFR measurement until two days after the STEMI treatment.
“You might consider IMR as a tool to stratify patients with heavy damage on the microcirculation… This means that there could indeed be a future role for a combined FFR plus IMR approach in this group of patients.”
Q. Research has shown that microcirculation can provide valuable prognostic information in acute myocardial infarction patients. Is there a place for the index of microcirculatory resistance (IMR) in this newly recommended treatment approach for STEMI patients?
Dr Engstrøm: Definitely, IMR could be very attractive and interesting. An increased IMR means that there is an increased resistance in the microcirculation and could indicate that the microcirculation is compromised. It corresponds to the size of the infarct and therefore may have a prognostic value. You might consider IMR as a tool to stratify patients with heavy damage on the microcirculation. In the acute phase, you can imagine that those patients are treated, for instance, with more aggressive medications or using alternative methods instead of just conventional primary PCI. There could be a future role for a combined FFR plus IMR approach in this group of patients.
Q. When you presented the PRIMULTI data around the world, what questions from your colleagues about the study or about treating this particular patient population came up that you did not anticipate?
Dr Engstrøm: One important criticism is the risk for unneeded repeat revascularisation. When a patient with an additional stenosis is randomised to the arm that doesn’t receive full revascularisation, the physician may be biased if the patient is re-admitted to the hospital afterward. This might lead to subsequent revascularisations that may not be needed. Having said that, we have looked into the two treatment groups and there was no difference between the two groups with regard to the frequency of readmission or the suspicion of angina. So we were able to address the question but we acknowledge there might be a bias.
Q. Where do we go from here in terms of understanding the need to assess the noninfarctrelated arteries during STEMI?
Dr Engstrøm: In a large-scale trial, we need to find out whether there is an effect of full revascularisation on hard endpoints. The Scandinavian FULL-REVASC (FFR-Guidance for ST Elevation Myocardial Infarction Revascularization) study will be initiated within the next six months. In this study, 4,000 patients will be randomised to FFR-guided full revascularisation versus revascularisation of the culprit lesion only. This study should be powered sufficiently to identify hard endpoints such as death and non-fatal myocardial infarction.
Thomas Engstrøm MD is currently head of interventional cardiology at Rigshospital in Copenhagen. Engstrøm studied at the University of Copenhagen and trained at Gentofte University Hospital, Skejby University Hospital and Rigshospitalet in Denmark. He is the author of more than 120 scientific articles in peer-reviewed journals.
Rx Only
Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.
Unless otherwise noted, ™ indicates that the name is a trademark of, or licensed to, St. Jude Medical or one of its subsidiaries. St. Jude Medical and the nine-squares symbol are trademarks and service marks of St. Jude Medical, Inc. and its related companies. © 2016 St. Jude Medical, Inc. All Rights Reserved.