The implantation of cardiovascular implantable electronic devices (CIEDs) including permanent pacemakers (PPMs), implantable cardioverter-defibrillators (ICDs) and cardiac resynchronisation therapy (CRT-D [with defibrillator] and CRT-P [with pacemaker]) devices are lifesaving procedures, and the expanding indications for device use have led to a marked increase in implantation procedures in recent decades. Advances in manufacturing technology have resulted in the availability of smaller, more sophisticated devices that are easier to implant. Furthermore, a growing body of evidence supports their beneficial effects on quality of life as well as potential costeffectiveness. 1 As a result of increased life expectancy, physicians will increasingly encounter patients with CIEDs.
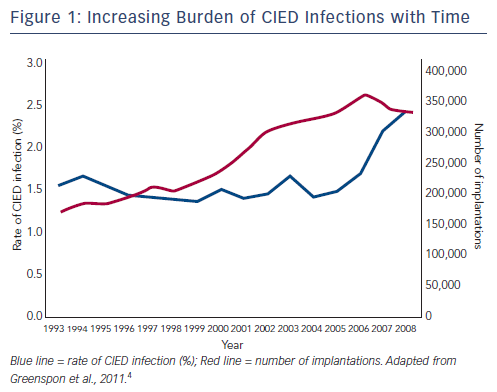
However, there is growing concern about the rate of infections in patients receiving CIEDs, since the incidence of CIED infection is increasing out of proportion to CIED implantation.2–4 A third of patients receiving CIEDs show signs of bacterial colonisation,5 some of which result in clinically evident CIED infection. A US study reported an increase in the number of CIED implantations from 199,516 in 2004 to 222,940 in 2006, a 12 % increment. However, in the same period, the number of CIED infections increased from 8,273 to 12,979, a 57 % increment.3 In another study, during the period 1993 to 2008, the incidence of CIED infection increased from 1.5 % to 2.41 %, with a marked increase noted in 2004, which coincided with an increase in the incidence of diabetes and renal failure among device recipients (see Figure 1).4
Numerous reasons for this rise in CIED infections have been proposed. More older patients are receiving CIEDs, and higher rates of comorbidity may predispose them to poor wound healing and diminished host immune defences.3,4,6,7 In addition, younger patients are receiving CIEDs, and an increasing number of patients are surviving long enough to require pulse generator changes and lead revisions,8 which are associated with a higher infection rate.9,10 Newer techniques are associated with longer procedure times: implantation of CRT-P requires careful placement of the left ventricular pacing lead in the optimal coronary sinus branch in order to optimise clinical benefit, extending case times by around 20–90 minutes and thus allowing more time for pocket or hardware contamination.11 There is also an increasing proportion of ICDs8 and dual chamber devices (DDD);7 ICDs are associated with higher infection rates than PPMs.8,12 Patients with an ICD have a higher prevalence of congestive heart failure and abdominal generator placement, and more undergo electrophysiological study prior to device implantation.13
CIED infections result in significant morbidity and mortality, frequently require device explantation and, if indicated, reimplantation.14–17 When subsequent care and re-implantation is considered, the cost may be substantially higher. Patients have longer durations of hospital stays and increased risk of in-hospital death.16 The rate of 1-year mortality following infection can be high (16.9 %), even after removal of the device,15 and was associated with a 1.9-fold increased risk of mortality compared with patients who did not experience CIED infection. After controlling for possible confounders, this represents a 2.4-fold increased risk of mortality.15 This results in a significant health and economic burden that may counteract the benefits of the devices; the total cost of an infection has been estimated at up to $53,000 per case, with intensive care accounting for almost half of the total cost.16 The annual cost of CIED infections in the US has been estimated at a minimum $285 million.18 The cost may, however, vary significantly depending on the type of healthcare system and the type of infection.
This article will review methods of management and prevention of CIED infections, with a focus on the TYRX™ Absorbable Antibacterial Envelope, which offers potential as a cost-effective method to reduce CIED infections.
Diagnosis and Management of CIED Infections
Diagnosis of CIED infection can be challenging, with symptoms ranging from local pocket erosion to full-blown sepsis.19 Clinical presentation includes symptoms such as erythema, warmth, tenderness, purulent discharge or erosion of generator or leads through the skin,20 and up to 30 % of patients present with nonspecific symptoms only, such as fever and malaise. Local pocket infections vary in onset and can occur more than a year after implantation.21 While diagnosis is straightforward in patients presenting with inflammatory conditions or lead erosion, differentiating between early postoperative haematoma and pocket infection is more difficult. Infections may be local or systemic; Staphylococcus infections are most common (60–80 % of reported cases) since Staphylococcus species are frequent colonisers of the skin and have the ability to produce a biofilm on the device surface, enabling them to evade host immune defences.22 Nonstaphylococcal infections are diverse and have a relatively low virulence and mortality rate.23 Recent data suggest a trend to more resistant organisms.8
Systemic infections, which include bacteraemia and lead-related endocarditis, also show wide variation in onset time; when divided into two groups, the late onset group had a mean onset of 31 months.24 Remote infection sites are seen in patients with late infection; late infection should therefore be considered in any CIED patient who presents with fever, bloodstream infection or signs of sepsis, even if the device pocket appears uninfected.25 It is essential to obtain at least two sets of blood cultures before the initiation of antimicrobial therapy in all patients with systemic infections since its presentation may be indolent;26 in patients with Staphylococcus aureus bacteraemia (SAB), positive blood cultures may be the only sign of infection.27 In cases of positive blood cultures, a transoesophageal echocardiogram (TOE) should be performed to evaluate for the possibility of underlying CIEDrelated endocarditis.26
Current management of CIED infection depends on the clinical presentation and the causative pathogen. Most CIED infections require removal of the whole system, and administration of IV antibiotics.28,29 Transvenous explantation is the preferred technique, but has been associated with a low rate of complications including haemothorax, laceration of the superior vena, damage to the tricuspid valve and cardiac tamponade.20,26 Satisfactory control of the infection is required before implantation of a replacement device may be considered.
Risk Factors for CIED Infections
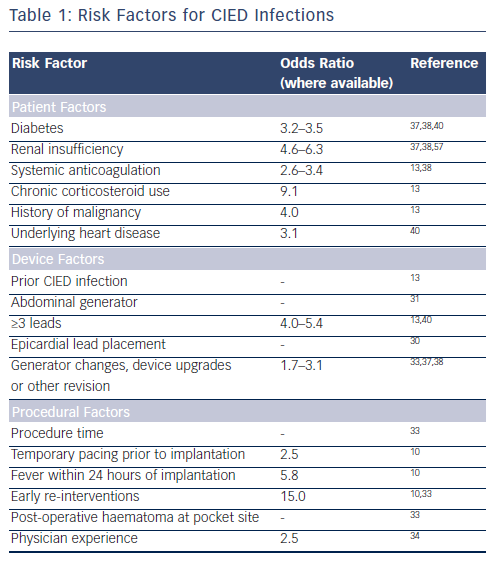
The rising rates and diagnostic challenges of CIED infections have led to numerous studies investigating risk factors for infection (see Table 1). Early CIEDs required the implantation of epicardial leads, which were associated with high infection rates.30 Subsequent advances have led to the majority of electrode leads being implanted via the transvenous route; epicardial lead placement usually occurs if transvenous implantation is not feasible. Likewise, studies on infection rates have led to pectoral implantation of generators rather than abdominal placement.31 Important device-related risk factors for infection include device revision or upgrade,32 the use of more than two pacing leads and the need for early pocket re-exploration.10,13 The presence of multiple leads increases the risk of central venous thrombosis in the area of the leads and is a potential site of secondary seeding of microorganisms.13 Procedure-related factors include: procedure time, temporary pacing prior to implantation, fever within 24-hour of implantation, early pocket re-entry and postoperative haematoma at the pocket site.33 In addition, physician experience is important; a study found a significantly higher risk of ICD infection within 90 days of implantation in patients whose device was implanted by physicians in the lowest quartile of procedural volume (odds ratio [OR] 2.47 compared with physicians in the highest-volume quartile).34
Patient factors that have been associated with an increased risk of CIED infections include younger age, male gender35,36 and renal insufficiency.8,37,38 The infection risk is particularly high in patients with renal failure undergoing chronic haemodialysis via an implanted central catheter. Such patients are at risk of recurrent bacteraemia from their dialysis catheter and subsequent seeding of the device leads and pulse generator.37 The use of anticoagulation therapy using warfarin is also associated with increased infection risk, possibly due to the increased risk of pocket haematoma, which may cause delayed wound healing or require surgical evacuation.13,38 Chronic use of immunosuppressant drugs such as corticosteroids is also a risk factor for CIED infection.13
Numerous other patient-related factors have been associated with increased risk of CIED infection, including a history of malignancy, underlying heart disease, fever within 24 hours of implantation and lack of antibiotic prophylaxis.10,20,33,38–40 Diabetes has been associated with surgical site infections among cardiothoracic surgery patients41 and has been identified with higher risk of CIED infection.37 The Centers for Disease Control and Prevention (CDC) have recommended adequate control of serum blood glucose levels in all diabetic patients and the avoidance of preoperative hyperglycaemia.42 A clinical risk score prior to implantation has been proposed: a recent study has developed a novel scoring index to risk stratify patients and proposed seven independent risk factors that predict infection.43 These comprise early pocket re-exploration, male sex, diabetes, upgrade procedure, heart failure, hypertension and glomerular filtration rate <60 mL/min. The study proposed a composite risk score (0–25; C index 0.72; 95 % confidence interval 0.61–0.83) using these seven factors: and identified three groups: low risk (score 0–7; 1 % infection), medium risk (score 8–14; 3.4 % infection), and high risk (score ≥15; 11.1 % infection).
While these studies provide a valuable insight into CIED infection risk factors, many are small, single-centre studies and there is a need for larger, more representative studies to identify the most important factors that are responsible for the development of CIED infection.
Strategies to Reduce CIED Infection
Numerous strategies to reduce CIED infection have been proposed. Adherence to aseptic techniques during implantation is important, and the use of chlorhexidine-alcohol for skin preparation and mupirocin nasal ointments have been reported.44,45 Pocket irrigation has been used but has not been proven to reduce infection risk. The antimicrobial treatment of pacemaker casings with antiseptics has also recently been investigated in vitro and early studies showed promising results.46 Other products have been investigated but have not been successful in preventing CIED infections. Collagen impregnated with antibiotic such gentamicin-impregnated collagen sponge (Collatamp G), has been used to prevent sternal wound infection in cardiac surgery,47 but there is no evidence to support its use in preventing CIED infections. A gentimycin-collagen fleece (Septocoll) has been used in other applications.48
To date, the only intervention proven in randomised clinical trials to reduce infections is intravenous prophylaxis using antibiotics.49 A double-blinded study included 1,000 consecutive patients who presented for CIED implantation or generator replacement. Patients were randomised to prophylactic antibiotics (intravenous administration of 1g of cefazolin immediately before the procedure) or placebo. The trial was terminated early after enrollment of 649 patients due to a significantly lower rate of infection rates in the antibiotic arm (0.63 % in antibiotic arm vs 3.28 %; RR 0.19; p=0.016).49 Vancomycin may be used in patients who are allergic to cephalosporins, have methicillinresistant Staphylococcus aureus (MRSA) colonisation or in institutions that have a high prevalence of MRSA infection.26
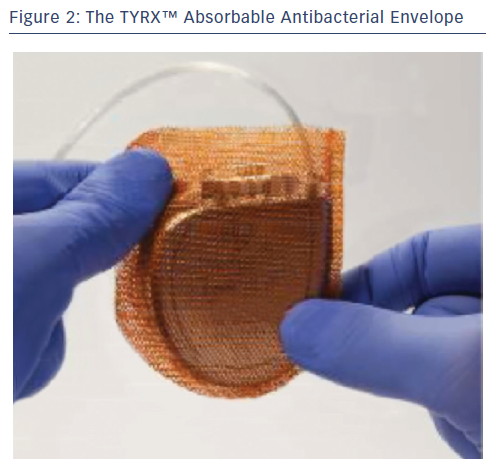
Pocket infection may be reduced using an antibiotic envelope. The TYRX™ Absorbable Antibacterial Envelope (formerly AIGISRx® R, Medtronic TYRX, Inc.) is constructed from a bioabsorbable multifilament knitted mesh polymer made of glycolide, caprolactone and trimethylene carbonate that is coated with a bioabsorbable polyarylate polymer and comprises two flat, rectangular sheets that are sealed on three sides (see Figure 2). The envelope holds the CIED in place, preventing CIED migration; elutes antimicrobial agents minocycline and rifampicin for a minimum of seven days; and then is fully absorbed approximately nine weeks after implantation. The device is available in two sizes: medium for PPMs and large for ICD/CRT devices. Implantation requires a subcutaneous pocket ~10 % larger than normal to accommodate the envelope. The TYRX Absorbable Antibacterial Envelope received US Food and Drug Administration (FDA) clearance in May 2013 and the CE Mark in September 2014.
Clinical Evidence Evaluating the Efficacy of the TYRX Antibacterial Envelope
At present, there are no published data on the TYRX Absorbable Envelope, but a substantial body of clinical data supports the efficacy of the previous generation non-absorbable envelope. The absorbable envelope has antibiotic efficacy identical to that of its predecessor and was regarded by the FDA as substantially equivalent, forming the basis for its approval. The efficacy of the non-absorbable antibacterial envelope has been evaluated in a preclinical study that demonstrated antimicrobial efficacy against Staphylococcus epidermidis, Staphylococcus capitis, Escherichia coli and Acinetobacter baumannii, as well as the elimination of biofilm on the implanted device.50
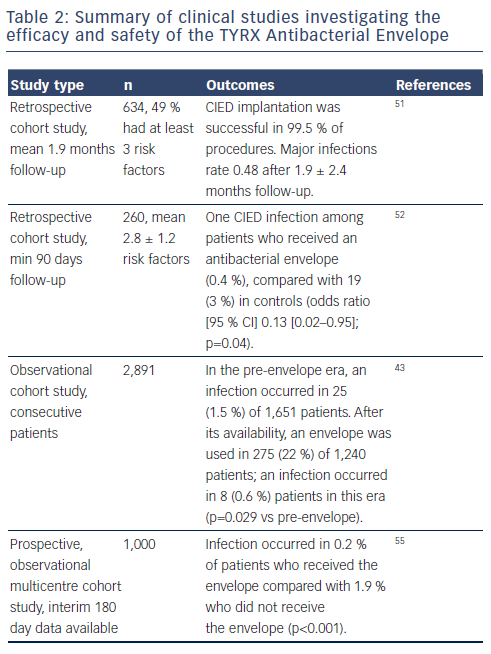
Clinical data describing the efficacy of the non-absorbable antibacterial envelope are summarised in Table 2. The COMMAND (Cooperative Multicenter Study Monitoring a CIED Antimicrobial Device) retrospective cohort study was conducted at 10 medical centres in the US and comprised 642 consecutive CIED patients who had undergone initial implantation or revision/replacement procedures utilising the antibacterial envelope. Almost half (49 %) had three or more predefined risk factors. The study reported only three major infections (0.48 %; 95 % confidence interval [CI] 0.17–1.40). The infections followed one ICD revision and two CRT-D replacements. There were seven deaths; none was a result of the antibacterial envelope or the CIED procedure. However, the follow-up was short at the time of reporting (1.9 ± 2.4 months).51 A retrospective single-centre cohort study included 260 patients presenting with at least two of the following risk factors within two weeks of original implantation: diabetes, renal insufficiency (creatinine ≥1.5 mg/dL 24 hours prior to implantation), systemic anticoagulation, chronic daily corticosteroid use, fever or leucocytosis 24 hours prior to implantation, prior documented CIED infection, or at least three transvenous leads. A historical control group (n = 639) was derived from a patient database. The study found a reduced rate of CEID infections after a minimum of 90 days follow-up among patients who received an antibacterial envelope (0.4 % compared with 3 %; odds ratio [OR] 0.13; 95 % CI 0.02–0.95; p=0.04).52
A recent study of the infection rate associated with use of the nonabsorbable envelope followed 2,891 consecutive patients for six months in the pre-envelope and post-envelope era.43 In the pre-envelope era, the infection rate among 1,651 patients was 1.5 %. In the post-infection era, an envelope was used in 275 (22 %) of 1,240 patients; an infection occurred in 0.6 % patients in this era (p=0.029 vs pre-envelope).
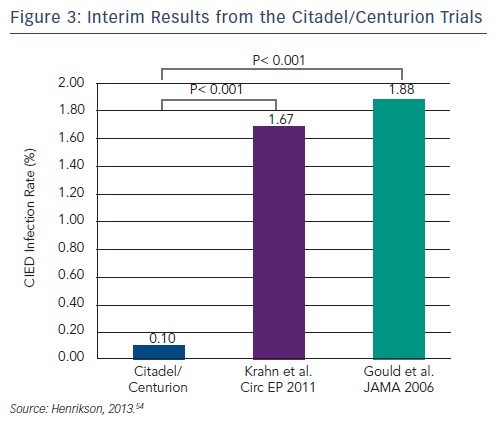
Two prospective multicentre cohort studies involving 66 US centres (n=1,000) are currently in progress. The Citadel study (TYRX Envelope for Prevention of Infection Following Replacement with an Implantable Cardioverter-Defibrillator) aims to compare the rate of CIED infection and mechanical complication after CIED replacement with an ICD and non-absorbable antibacterial envelope, to that after replacement with an ICD and no antibacterial envelope (NCT01043861). The study population in the Centurion trial (TYRX Envelope for Prevention of Infection Following Replacement With a Cardiac Resynchronization Therapy Device) comprises patients who have undergone CIED replacement with a CRT (NCT01043705). The historical control group in both trials consists of 533 Canadian patients in a retrospective study, all of whom underwent CIED replacement because of device advisories or recalls.53 The major device infection rate in this study during a mean 2.7 months following device replacement was 1.9 %, compared with 0.1 % in 90-day data reported in Citadel/Centurion (see Figure 3).54 The chief device-related complication recorded during the study was major pocket haematoma in 1.5 % of patients who received the envelope vs 2.25 % in the historical controls. This required a change in patient management, such as open drainage or transfusion. More recently presented interim data showed that the low infection rate was maintained at 180 days.55 Infection occurred in 0.2 % of patients who received the envelope compared with 1.9 % who did not receive the envelope (p<0.001). In addition, there was a low rate of device mechanical complications (4.0 %) in patients who received the envelope.
Currently there are no published data on the effectiveness of the newer fully absorbable version of the antibacterial envelope. The WRAP-IT (Worldwide Randomized Antibiotic Envelope Infection Prevention Trial) is a multicentre, single blinded randomised study and is currently recruiting in over 200 clinical sites worldwide.56 This study will evaluate the ability of the TYRX Absorbable Envelope to reduce major CIED infections during 12 months following CIED generator replacement, upgrade, revision or de novo CRT-D implant. Patients (n=6,988) will be randomised 1:1 to envelope versus no envelope. Its primary endpoint is the rate of major CIED infections through 12 months resulting in one or more of the following: CIED system removal, CIED pocket revision, CIED infection treated with antibiotic therapy if the subject is not a candidate for system removal and death due to CIED infection. Secondary objectives include all cause mortality within 12 months and CIED removal due to pain without any clear infection. The estimated study completion date is December 2017.
Discussion and Concluding Remarks
Despite advances in the understanding of the pathogenesis, risk factors and management of CIED infections, the infection rate is rising faster than the rate of implantation of CIEDs, representing an important, expensive and potentially preventable complication of device implantation. The TYRX Absorbable Antibacterial Envelope represents a significant clinical advance. Since the TYRX Absorbable Antibacterial Envelope only recently received its CE mark, the author’s early experience is limited but suggests that it is easy to use and is associated with a very low early complication rate.
The major disadvantage of the envelope is the cost. It is important to select patients for implantation according to risk of CIED infection. However, given the substantial expense associated with infections, it is hoped that the use of the envelope in high-risk patients will prove cost-effective in the long-term. A 2011 report (based on the TYRX Non-absorbable Envelope) suggested that the estimated absolute risk reduction associated with the use of the antibacterial envelope is 2.5 %, generating a number of 40 patients needed to treat to prevent one infection.
In conclusion, use of the TYRX Absorbable Antibacterial Envelope appears to be a promising strategy with the potential to reduce CIED infections, but clinical experience is limited. There is a need for large, multicentre, randomised data to fully establish the clinical benefits and cost effectiveness of the absorbable antibacterial envelope. The results of the ongoing WRAP-IT trial are eagerly awaited.