With an ageing population, the burden of peripheral artery diseases (PADs) is increasing. The treatment of these diseases has largely been performed by interventional radiologists, vascular surgeons and interventional cardiologists. In 2011, the percentage of procedures performed per specialty were 12.9 %, 45.0 % and 42.2 % for interventional radiology, vascular surgery and interventional cardiology, respectively.1 Due to the complexity of the disease and comorbidities of the patients with peripheral arterial disease, a multidisciplinary collaborative approach between these subspecialties is important for the best outcomes.
Although there are rare atypical forms of PAD, it shares the same traditional risk factors for atherosclerotic disease; namely, smoking, diabetes mellitus, high blood pressure, dyslipidaemia and older age. Large registries have shown that 50–60 % of patients with PAD have cerebrovascular or coronary artery disease, and patients with PAD have a two-fold increase risk of all-cause mortality compared with those of matched Framingham risk scores with no PAD.2 The physician who cares for the patient with peripheral vascular disease should have a broad understanding of atherosclerotic disease involving all vascular beds.
Although endovascular interventions play a major role in relieving symptoms and reducing the morbidity associated with PAD, longterm optimal medical treatment is an essential determinant of prognosis. The cornerstones to medical therapy are 3-hydroxy- 3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors and antithrombotic therapy. Although randomised trial data are lacking with regard to the exact length of dual antiplatelet therapy with aspirin and clopidogrel, it does appear that short duration combination therapy is important following peripheral interventions. During the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial, 15,603 patients were randomised to either clopidogrel plus low-dose aspirin or low-dose aspirin plus placebo. Dual antiplatelet therapy did not reduce the primary endpoints of death, myocardial infarction or cardiovascular death; however, it was associated with an increase in moderate bleeding.3 For iliac, femoropopliteal and drug-eluting stents, the current recommendations for dual antiplatelet therapy are 1 month, 1–3 months and 2 months, respectively.4 In situations such as stent graft repair, covered stents and poor outflow, long durations of dual antiplatelet therapy can be considered if the bleeding risk is low.
This paper reviews current endovascular/percutaneous interventions for PAD.
Endovascular Aortic Repair
Abdominal Aortic Aneurysm
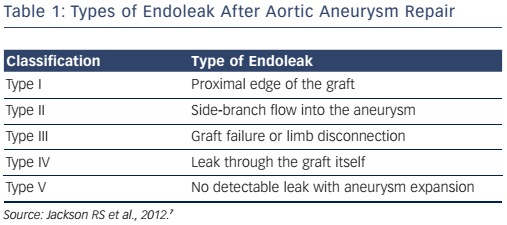
A ruptured aortic aneurysm carries a mortality rate >75 % and is the 13th leading cause of death in the USA.5 Routine screening is recommended in high-risk patients and repair should be considered when the aneurysm’s maximal diameter reaches 5.0–5.5 cm (varies by guidelines).6 Endovascular aneurysm repair (EVAR) using stent grafts is a popular treatment option for patients with abdominal aortic aneurysm. Due to favourable outcomes over open surgical repair, in current practice, endovascular repair accounts for more than 80 % of all cases of abdominal aortic aneurysm repair performed in the USA.7 Clinical trials comparing EVAR to open surgical repair demonstrate a significantly lower peri-procedural mortality and morbidity with EVAR but similar long-term mortality and a higher risk of repeat interventions due to graft endoleaks (see Table 1).8
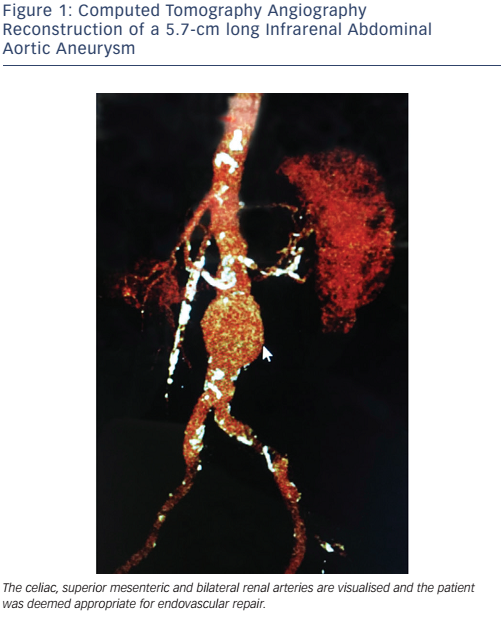
EVAR is limited to the patients who meet the anatomic criteria for the stent graft device available. Most devices require an adequate proximal landing zone (aneurysm neck) that is 10–15 mm long and free of significant angulation or calcification. Newer devices have a smaller profile and a more flexible delivery system that are useful in patients with difficult iliac and common femoral anatomy. As an alternative to performing a femoral artery cutdown for vascular access, which requires surgical repair at the end of the procedure, many operators utilise the ’pre-close technique’9 for access site management, allowing for EVAR to be done entirely percutaneously. Figure 1 shows a computed tomography angiography (CTA) reconstruction of an infrarenal aortic aneurysm in a patient who presented for consideration of endovascular repair.
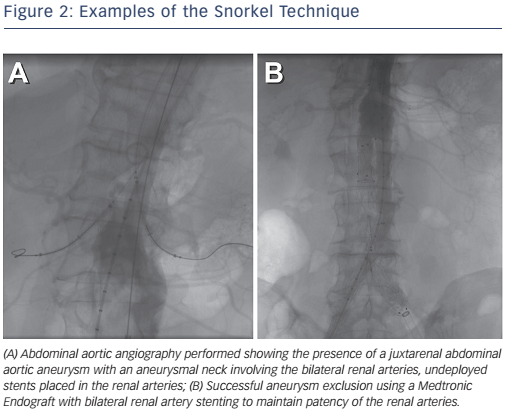
Advanced techniques have been used for junta-renal aneurysms with poor landing zones that combine renal stenting with EVAR to maintain renal perfusion (see Figure 2).10 Fenestrated and branched grafts are also available to maintain renal and mesenteric perfusion in complex aneurysms, but their long-term outcomes remain to be demonstrated.
Descending Thoracic Aorta
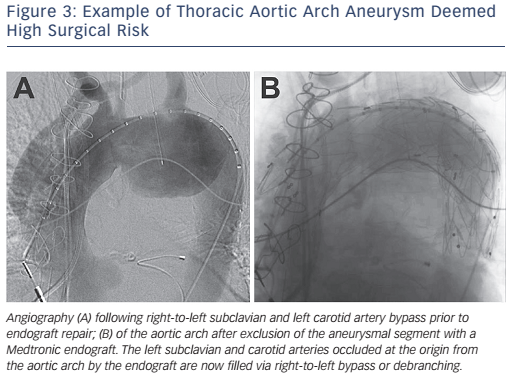
With current endovascular techniques and stent graft design, endovascular repair is the current treatment of choice for aneurysmal disease of the descending thoracic aorta that meet criteria for repair (see Figure 3).11 Contemporary trials comparing endovascular with surgical repair of descending thoracic aortic aneurysms have shown a significantly lower mortality (5.8 versus 13.9 %; p<0.001), stroke and paraplegia (8.9 versus 18.7 %; p<0.001), and other preoperative complications in patients treated with endovascular repair without increased risk of repeat interventions.12 With the improved outcomes seen with endovascular repair of the descending thoracic aortic aneurysms, this treatment has now been extended to Stanford type B acute aortic dissections. In the Investigation of Stent-grafts in Aortic Dissection (INSTEAD-XL) trial, endovascular repair was shown to improve aortic remodelling and decrease long-term vascular complications and mortality better than medical treatment alone in uncomplicated type B aortic dissections.13
Lower Extremity Peripheral Arterial Disease
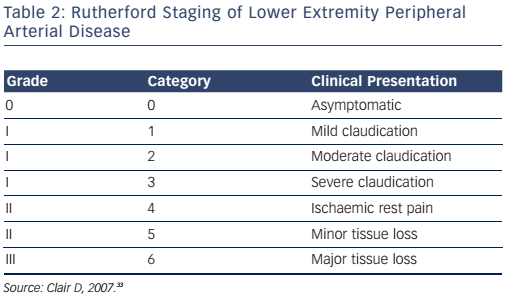
The lower extremity is the most commonly affected arterial bed in patients with peripheral vascular disease. The Rutherford staging system (see Table 2) is used to classify the stages of lower extremity peripheral arterial disease. Categories 4, 5 and 6 constitute critical limb ischaemia. The goals of therapy for lower extremity arterial disease are to prevent cardiovascular morbidity and mortality, relieve symptoms and to preserve the limb. Medical therapy involves antiplatelet therapy with aspirin, Hmg-CoA reductase inhibitors, smoking cessation and control of diabetes and hypertension. Cilostazol may be added to reduce symptoms of intermittent claudication but its use is contraindicated in patients with congestive heart failure. A supervised walking exercise programme is recommended to promote collateral circulation and improve symptoms of claudication.
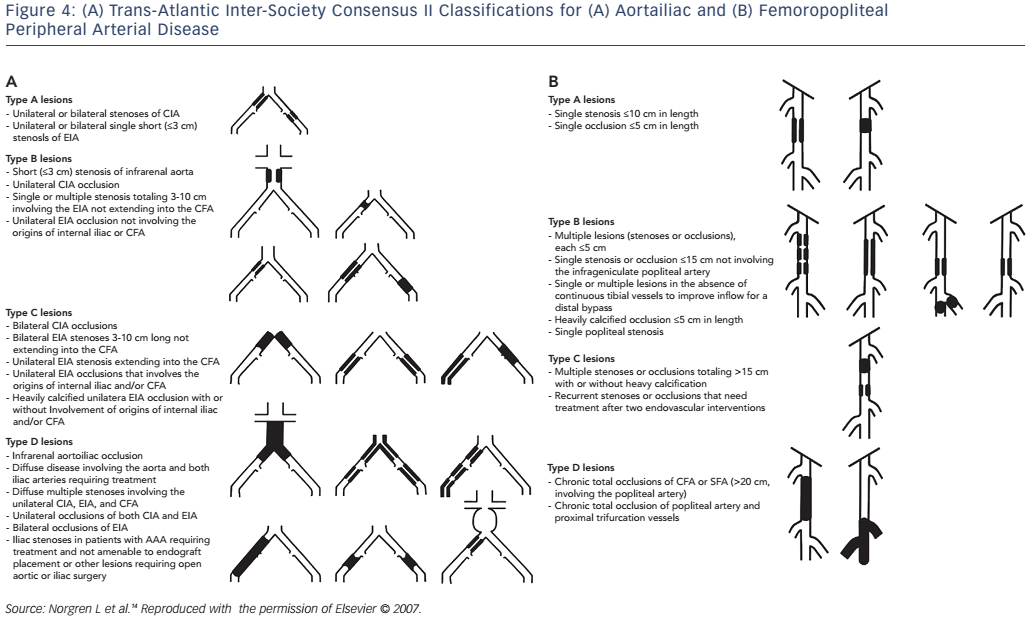
Revascularisation is recommended in patients with significant claudication refractory to medical therapy, critical limb ischaemia (rest pain, non-healing ulcer or gangrene) or acute limb ischaemia. The choice of revascularisation strategy is largely based on the likelihood of technical success and long-term vessel patency. The Trans-Atlantic Inter-Society Consensus (TASC) II classification (see Figure 4) is used to denote the anatomic severity of disease and correlates with the likelihood of endovascular procedural success.14 Generally speaking, endovascular intervention is recommended for TASC A and B lesions while surgical revascularisation is the treatment of choice for TASC D lesions in patients who are not of high surgical risk.
Supra-inguinal Arterial Disease
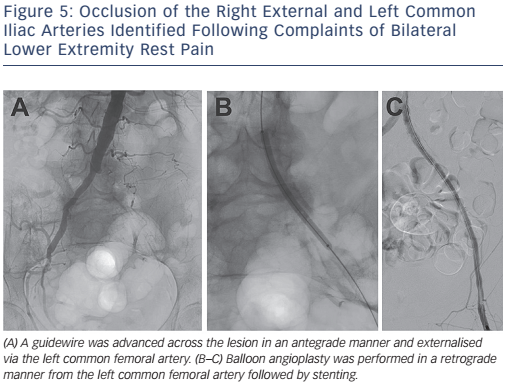
For a disease involving the aortoiliac system, percutaneous angioplasty and stenting is considered the treatment of choice for focal, TASC A and B lesions. Treatment of such lesions has a high procedural success rate with 5-year patency rates of approximately 80 % with lower morbidity than surgical revascularisation. Surgical revascularisation is considered the treatment of choice for TASC C and D lesions; however, given the high-risk nature of many patients, endovascular therapy is being considered in this population with improvements in long-term outcomes. Figure 5 demonstrates a TASC D lesion (unilateral occlusion of the common and external iliac artery); however, the patient shown in this image was considered a high surgical risk and therefore underwent endovascular intervention.
Infra-inguinal Arterial Disease
The superficial femoral and popliteal arteries are the most common targets for intervention in PAD. Available treatment options for femoropopliteal disease include standard balloon angioplasty, selfexpanding nitinol stents, covered stents, atherectomy, drug-eluting stents and, most recently, drug-coated balloons (DCBs).
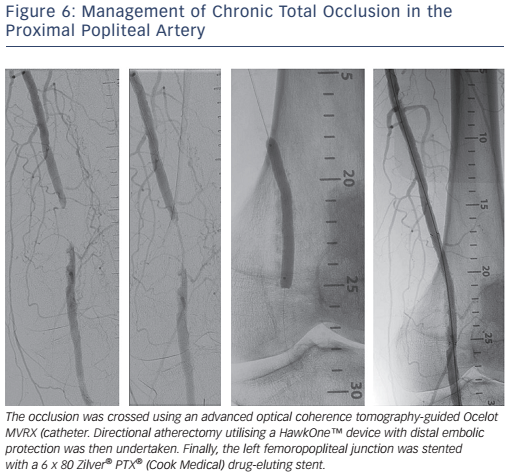
Conventional endovascular treatment of femoropopliteal disease has relied on balloon angioplasty with provisional stenting used if there is >50 % residual stenosis, a significant residual pressure gradient or a flow-limiting dissection. With advancements in stent design, including the use of nitinol self-expanding stents, routine stenting is a viable long-term option (see Figure 6). The treatment of long lesions >150 mm with self-expanding stents is however associated with worse long-term patency.
The use of drug-eluting stents and DCBs may help to improve long-term patency rates of femoropopliteal lesions. The 5-year patency rate for superficial femoral artery (SFA) lesions with the Zilver® (paclitaxil) drug-eluting stent has been shown to be superior to balloon angioplasty with provisional bare-metal stenting (66.4 versus 43.4 %).15 Similarly, the use of the In.PACT® (Medtronic Minneapolis, MN, US) DCB has also been shown to have superior primary patency over standard percutaneous transluminal angioplasty (PTA) alone (78.9 versus 50.1 %; p<0.001) at 2 years. The rates of target lesion revascularisation (TLR) were 9.1 and 28.3 %, respectively (p<0.001).16 The attraction of DCB is in avoiding stent failure related to mechanical stress in the superficial femoral and popliteal arteries. DCBs have also shown higher primary patency, clinical symptom improvement and lower TLR than standard balloon angioplasty in the treatment of femoropopliteal in-stent restenosis.17
Infra-popliteal Disease
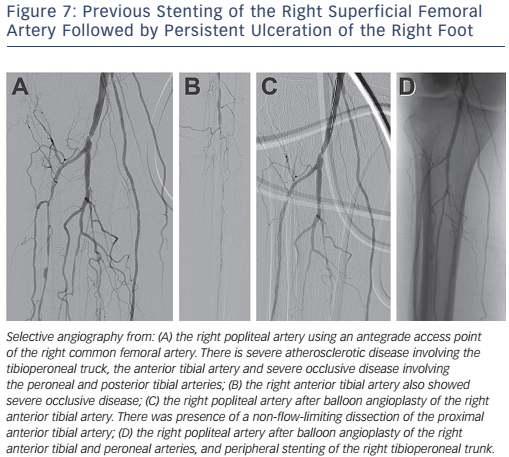
Revascularisation for infra-popliteal disease is largely indicated for patients with critical limb ischaemia to prevent or limit limb loss. In addition to medical treatment, endovascular intervention largely consists of balloon angioplasty with stenting being performed as a bailout strategy (see Figure 7). Although DCBs have shown promising results in femoropopliteal disease, the Study of IN.PACT Amphirion™ Drug Eluting Balloon vs. Standard PTA for the Treatment of Below the Knee Critical Limb Ischemia (In.PACT DEEP) trial for infrapopliteal disease did not show benefit on primary patency or TLR over standard balloon angioplasty (TLR 9.2 versus 13.1 %; p=0.291).18
Drug-eluting stents are increasingly used as the primary strategy for revascularisation in infrapopliteal lesions. In a meta-analysis of 611 patients from five randomised controlled trials, drug-eluting stents reduced TLR (OR 0.31; 95 % CI [0.18–0.54]; p<0.001), restenosis (OR 0.25; 95 % CI [0.15–0.43]; p<0.001) and amputation (OR 0.81; 95 % CI [0.26–0.97]; p=0.04) more effectively than balloon angioplasty or baremetal stents.19 Most recently, results of wound healing and quality of life from the Comparing Angioplasty and Drug-eluting Stents in the Treatment of Subjects With Ischemic Infrapopliteal Arterial Disease (ACHILLES) trial have shown that stenting is a viable treatment option. In this recent review, 109 open wounds were assigned to PTA or stenting with sirolimus-eluting stents. At 6 months, wound volume reduction was higher in the stenting group compared with PTA alone (95 versus 60 %; p=0.048). At 1-year, there was also a trend toward higher rate of complete wound healing in the sirolimus-eluting stents group compared with the PTA group (72.9 versus 55.6 %; p=0.088).20
Surgical Treatment for Peripheral Artery Disease
While TASC type A lesions are optimal for percutaneous revascularisation, TASC type D lesions typically require surgical revascularisation. TASC B and C lesions require more careful consideration of the risks and benefits of percutaneous versus surgical revascularisation, patient comorbidities and technical abilities of the operators. When surgical revascularisation is required, above the knee anastomosis as well as conduit type, vein versus polytetrafluoroethylene (PTFE), are important for long-term patency. For above the knee bypass, the 4-year patency rate of vein conduit versus PTFE was 69 % and 60 %, respectively. In below the knee bypass, the 4-year patency rate has been found to be 77 versus 40 % for vein graft and PTFE grafts, respectively.21 For infrapopliteal disease, the 4-year patency rates are even lower at 62 % and 21 % for saphenous vein graft and PTFE, respectively.21 For best long-term outcomes, the anastomotic site should preferentially be above the knee and saphenous vein grafts are the conduit of choice.
Carotid Artery Disease
Stroke is the fifth leading cause of death in the USA; approximately 800,000 people suffer a stroke each year.22 Although carotid artery dissection, trauma and arteritis can result in cerebrovascular ischaemia, atherosclerosis is the most common cause of disease involving the internal carotid artery. Smoking cessation and medical therapy, including antiplatelet therapy and Hmg-CoA reductase inhibitors (statins), are the foundation of treatment for stroke and transient ischaemic attacks. Antiplatelet therapy with aspirin alone (75–325 mg), clopidogrel (75 mg) or the combination of aspirin plus extendedrelease dipyridamole (25 and 200 mg twice-daily) is recommended.23
Carotid endarterectomy (CEA) is the gold standard of revascularisation for carotid occlusive disease, but carotid artery stenting (CAS) is a good alternative in select cases.23 In the two contemporary trials, Carotid Revascularization Endarterectomy vs Stenting Trial (CREST)24 and Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE),25 involving CEA versus CAS, the rate of composite primary endpoints (periprocedural stroke, myocardial infarction, death or post-procedural ipsilateral stroke) were similar among the two groups. The risk of periprocedural stroke may be higher in the CAS group and the risk of myocardial infarction is higher in the CEA group. A recent meta-analysis of CAS versus endarterectomy among symptomatic patients with carotid stenosis showed that increasing age was associated with significantly higher likelihood for stroke within 120 days in patients treated with CAS, but age had no effect on periprocedural stroke after CEA.26 Head-to-head comparison between CAS and CEA showed no difference in periprocedural strokes in patients younger than 70 years, but in those 70 and older CAS was associated with a two-fold increase in periprocedural strokes over CEA. After 120 days there was no difference in new ipsilateral strokes between CAS and CEA.
CEA is recommended in patients of average to low surgical risk (see Table 3) who experience a non-disabling ischaemic stroke or transient ischaemic attack including hemispheric events or amaurosis fugax within 6 months (symptomatic patients) who have at least a 70 % stenosis on non-invasive imaging or more than 50 % on angiography.20 CAS is indicated as an alternative to CEA for symptomatic patients who are considered to be at high surgical risk.27
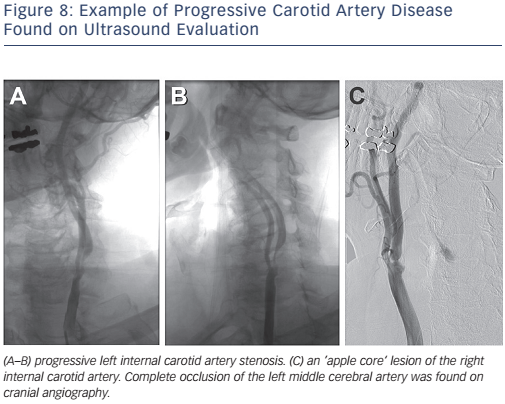
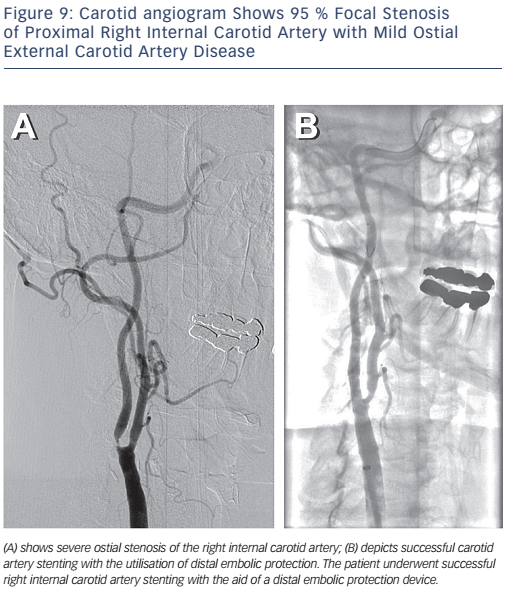
The Centers for Medicare and Medicaid Services (CMS) has concluded that CAS with embolic protection is reasonable in patients at high risk for CEA who have symptomatic carotid stenosis >70 %. Patients who are at high risk for CEA may be considered for CAS if they are symptomatic with stenosis >50–70 % or asymptomatic with stenosis >80 % as part of an approved trial or post-marketing study.28Figure 8 depicts bilateral carotid angiography where Figure 9 shows successful CAS with the use of an embolic protection device.
Subclavian
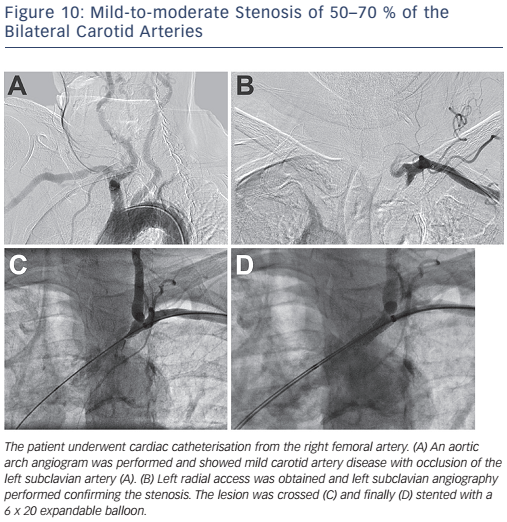
Upper extremity occlusive disease accounts for only 5–6 % of all cases of limb ischaemia.5 Subclavian artery stenosis is most commonly the result of atherosclerotic disease involving the proximal portion of the vessel. The region of stenosis typically occurs proximal to the vertebral artery, which can result in the subclavian steal phenomenon. Subclavian steal occurs secondary to subclavian stenosis with resultant reversal of flow in the ipsilateral vertebral artery. Although much of subclavian stenosis is asymptomatic, patients may present with upper extremity claudication as well as syncope (see Figure 10).
Two special populations involving patients with subclavian stenosis are those who have undergone coronary artery bypass grafting with an internal mammary bypass graft or the patient who has undergone lower extremity bypass grafting with an axillofemoral bypass. In these patients, symptoms can be consistent with classic angina, in the patient with coronary-subclavian steal phenomena, or lower extremity claudication in the patient who underwent peripheral artery bypass grafting. Subclavian artery intervention via the surgical or percutaneous approach should be considered in patients with symptomatic severe ischaemia.23
Renal
The prevalence of renal artery stenosis (RAS) has been estimated at approximately 2 % in unselected hypertensive patients versus up to >40 % in patients older than 55 years with risk factors for atherosclerotic disease.29 Atherosclerotic disease of the renal arteries typically involves the ostium and proximal one-third of the vessel. Stenosis of the renal arteries results in activation of the reninangiotensin-aldosterone system, which can lead to vasoconstriction, sodium and water retention (via aldosterone secretion), sympathetic nervous system activation, remodelling and ultimately hypertension.30
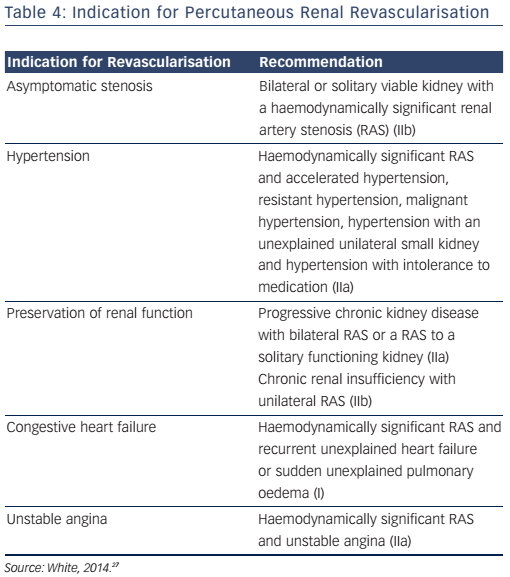
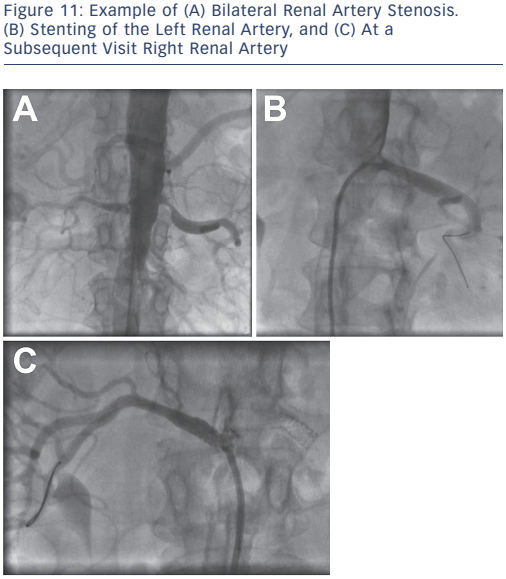
The first-line management of renal vascular disease involves medical treatment for secondary prevention of atherosclerotic disease and optimisation of anti-hypertensive therapy. Although clinical trials for renal artery revascularisation continue to show no improvement over optimal medical therapy, these trials have been subject to major design flaws including patient selection, lesion severity and sample size. Current recommendations for endovascular treatment of renal artery stenosis include stenting for ostial atherosclerotic disease (see Figure 11) in patients that meet the criteria for intervention (see Table 4) and balloon angioplasty with bailout stenting for fibromuscular dysplasia.29
Celiac and Mesenteric Arteries
Diseases of the mesenteric arteries (celiac, superior mesenteric, inferior mesenteric arteries) typically occur in patients with diseases in other vascular beds. Although atherosclerotic disease and thrombotic occlusion are the common causes of ischaemia due to the extensive collateral network, symptoms of ischaemia rarely present until there is disease involving multiple main mesenteric arteries.
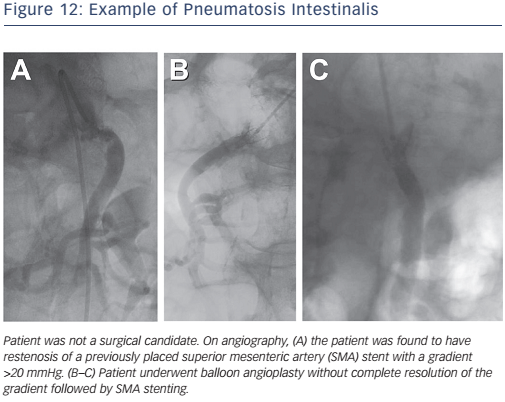
Acute mesenteric ischaemia is a medical emergency. It is typically the result of an embolism from the heart, atherosclerotic embolism during vascular procedures, acute thrombotic occlusion or diffuse ischaemic injury during profound shock. Treatment for acute intestinal ischaemia involves laparotomy, revascularisation of the ischaemic segment and assessment for viability and resection of non-viable bowel segments. Current options for endovascular treatment of acute intestinal ischaemia include transcatheter thrombolytic therapy, balloon angioplasty and stenting (see Figure 12).31
Chronic intestinal ischaemia is usually caused by atherosclerosis but unusual aetiologies include giant cell arteritis, Takayasu arteritis, fibromuscular dysplasia or extrinsic compression. The classic presentation is abdominal pain after eating (‘intestinal angina’). Weight loss occurs owing to the avoidance of food. Due to the lower procedural morbidity and good technical success with endovascular revascularisation, it has become the preferred treatment when feasible over surgical revascularisation by endarterectomy or bypass grafting.32 In chronic intestinal ischaemia, both surgical and endovascular therapies have received a level B I indication.31
Conclusion
As the population continues to age, patients are at an increased risk for atherosclerotic disease involving all vascular beds including the coronary, cerebral and peripheral circulation. The evaluation and management of patients with all forms of vascular disease and the expansion of options for endovascular therapies has increased the scope of practice for interventional cardiology. As technologies for endovascular devices continue to advance, new therapeutic options and improved clinical outcomes are expected.