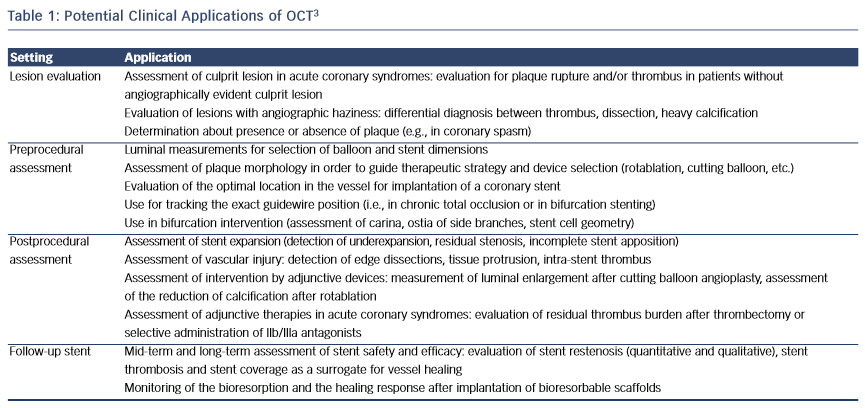
Intracoronary optical coherence tomography (OCT) is a light-based imaging modality able to visualise with high resolution (~10 μm) the vascular morphology and the acute and chronic effects of intervention with intracoronary devices.1,2 OCT could therefore find application in the guidance of percutaneous coronary intervention (PCI), allowing a thorough preprocedural lesion assessment, which enables accurate device sizing, selection of the vessel segment requiring treatment, and, thus, efficient planning of the implantation strategy (see Table 1).3 Moreover, it can be used for the assessment of the acute procedural result, allowing the estimation of stent expansion and vessel injury. Consequently, intravascular imaging can in this way assist in the optimisation of the acute implantation result, the significance of which is underscored by observations of an association between suboptimal implantation and stent failure.4 Importantly, several studies and meta-analyses have shown that the use of imaging guidance might improve outcome.5–7
Although OCT can provide a high amount of detail in the assessment of coronary arteries, this information might be challenging to be directly applied in the guidance of interventions in the daily cath lab practice, which are performed using real-time fluoroscopic guidance. This could happen because the spatial correspondence of OCT findings with the vessel angiogram is not always straightforward as a result of vessel overlap, foreshortening or the inability to visualise a complex three-dimensional (3D) structure correctly in a two-dimensional (2D) image. As the operator is using the angiographic image as a guide for performing the intervention, it is essential for him or her to ensure correct spatial orientation of the invasive imaging findings with the angiogram. Such problems might become more exaggerated in cases with diffuse disease, angiographically silent lesions or in the absence of side branches that can function as landmarks. In order to overcome this problem, approaches implementing an online co-registration of OCT with the coronary angiogram, which allows the operator to scroll through a synchronised dataset, would be highly desired. Such information could be useful for procedural planning and longitudinal assessment of atherosclerotic lesions.
We present a new technology allowing the online co-registration of OCT images with the angiogram in the catheterisation laboratory and discuss its potential utility in optimising procedural outcome in everyday practice.
OPTISTM Integrated System – A New OCT Imaging System with Online Angiographic Co-registration
The OPTISTM integrated system (St. Jude Medical, St Paul, MN, US) is an OCT imaging system that is integrated in the catheterisation laboratory and has the additional ability to provide a real-time co-registration of OCT images with the angiogram of the studied vessel. The OPTIS integrated system can be directly installed in a catheterisation laboratory and consists of the OCT imaging system, a pullback device, monitors and a tableside controller, while image acquisition is being performed with the DragonflyTM Duo (St. Jude Medical) OCT imaging catheter.
The imaging system is located within the control room of the catheterisation laboratory, while OCT data are projected in real time on a screen within the intervention room. Angiographic data from the existing angiographic system are retrieved and displayed simultaneously on the same screen. The pullback device is stowed in a holster positioned at the cath lab table. Direct tableside controls at the cath lab table allow for an autonomous handling of the system by the operating physician without the need for extra cable connections as in systems based on mobile carts. The OCT system specifications are similar to the previously used ILUMIENTM OPTISTM PCI Optimization system (St. Jude Medical) allowing for acquisition of OCT images with a frame rate of 180 frames per second. The pullback speed can be adjusted by the operator in one of two settings:
- A Survey mode with a 75 mm long pullback and a frame density of 5 frames per mm, which finds application in the assessment of longer coronary segments; and
- A High Resolution mode with a 54 mm long pullback and a frame density of 10 frames per mm, which can provide a more detailed longitudinal assessment of the vessel and stent morphology.
The Dragonfly Duo imaging catheter is a 2.7 F, rapid exchange monorail catheter with a pullback speed up to 75 mm/sec. Further, the distal catheter end carries a number of markers: a distal radiopaque marker indicating the guidewire exit, a proximal marker that indicates the ending position of the pullback and a marker indicating the position of the OCT lens. This marker moves simultaneously with the lens and allows the real-time tracking of its position by fluoroscopy. Finally, a femoral shaft guide marker has been added to identify when the Dragonfly Duo catheter is exiting a 100 cm guide catheter.
A number of options for displaying the OCT information are available. These include an automated lumen profile, which is generated after automated lumen detection in all OCT cross-sectional images throughout the pullback. This view provides information regarding the mean luminal diameter along the pullback, thus enabling a luminographic assessment of the studied vessel, but with the use of OCT measurements, overcoming potential limitations of angiography in luminal assessment such as foreshortening, vessel overlap or filling artifacts.
Information such as minimal lumen diameter, reference diameters and degree of area or diameter stenosis are simultaneously projected on the screen, thus providing a fast and practical assessment of the severity of luminal stenosis, while giving important information for sizing. Moreover, OCT information is also reconstructed in 3D and can be displayed in parallel. This visualisation might aid in the assessment of bifurcations or in cases with stent deformation.
The angiographic image that is recorded during the pullback is projected together with the OCT image. After semi-automatic co-registration, a small white marker is projected over the angiogram, indicating the exact location of the displayed OCT frame on the angiogram. This information is useful for the direct utilisation of intravascular imaging findings in procedural decision making, as the location of the OCT images is directly displayed and facilitates the selection of a suitable landing zone for the intervention.
Clinical Relevance of Online OCT Co-registration
Overall, the use of a system able to provide online spatial co-registration of the high-resolution intravascular imaging findings with the angiographic image could improve decision-making in the cath lab. This integration of OCT information on an angiographic roadmap enables the easy and immediate utilisation of such information by the operator. This could find broad application in the treatment of complex or diffuse disease, where spatial orientation might be challenging, requiring continuous fluoroscopy and multiple views in order to correctly localise the segment that needs to be treated.
The advantage of using a co-registered OCT approach might be even more pronounced in the case of bioresorbable scaffold implantation. As the current designs of bioresorbable scaffolds have relatively thick struts and high crossing profiles, an extensive lesion preparation is required in order to be able to advance the device to the site of the lesion and achieve an optimal implantation result. An accurate delineation of the required landing zone is mandatory in order to avoid problems such as a mismatch between lumen scaffold dimensions or incomplete lesion coverage. Mismatch of lumen and scaffold dimensions should be avoided considering the narrow postdilation limits of bioresorbable scaffolds, where expansion above the recommended limits has been associated with fracture.8 Furthermore, in view of the need for extended vessel preparation with increased incidence of predilation, vessel injury might be more pronounced in comparison with metallic devices, where direct implantation is usually preferred. This has important clinical implications, as incomplete lesion coverage has been associated with bioresorbable scaffold failure.9,10 Therefore, a complete coverage by the scaffold of the segment subjected to predilation is desired, and the co-registration of the angiogram with OCT images providing information regarding the injured and healthy vessel wall can aid in ensuring this optimal coverage.
Another important field where OCT can provide useful guidance in clinical practice is in the management of stent failure, where the recent European Society of Cardiology guidelines have given OCT a class IIa recommendation (level of evidence: C).11 In acute and subacute stent thrombosis, mechanical factors such as incomplete expansion and vessel trauma play a pivotal role.5 It is important to recognise these mechanical complications in order to provide the appropriate treatment (e.g., postdilation in incomplete expansion or additional stent implantation in edge injury). The knowledge of the precise anatomical location can facilitate local treatment, especially in long stents or stents with asymmetric expansion, where the exact localisation of the site with the mechanical issue might be poorly visualised by angiography. Also, in late stent failure, the distinction of restenosis with thrombosis might be unclear by angiography,12 while use of OCT can help discriminate between these two mechanisms, and guide the choice between local or systematic antithrombotic therapy, balloon postdilation or additional stent implantation. Again, the localisation of the stent pathology is important, as the severity and extent of restenotic tissue and/or thrombus could vary, while the visualisation by the angiography remains poor. In such cases, co-registered OCT could allow treatment that is focused on treating the proper segment within the stent.
Overall, in our practice, OCT is being frequently used in the preprocedural lesion assessment providing accurate measurements for stent or scaffold sizing, aiding in the choice of the interventional strategy and in the delineation of a suitable landing zone. According to our experience, the use of a co-registered OCT system often facilitates decision making in a way readily and easily available, without obstructing the workflow of the laboratory. The integration of structural OCT information into the angiographic luminogram provides the desired angiographic landmarks that indicate the desired segment for positioning of the stent or the balloon. This finds application also for the postprocedural assessment where co-registration has proved to be useful in precisely localising regions with marked malapposition or incomplete expansion and treating appropriately. This strategy can help ensure an optimal implantation result, with adequate device expansion and apposition and minimisation of vessel injury. The following cases describe how co-registered OCT can be used in daily practice to improve outcomes.
Case study 1. OCT-guided BVS Implantation in a Patient with Acute Coronary Syndrome
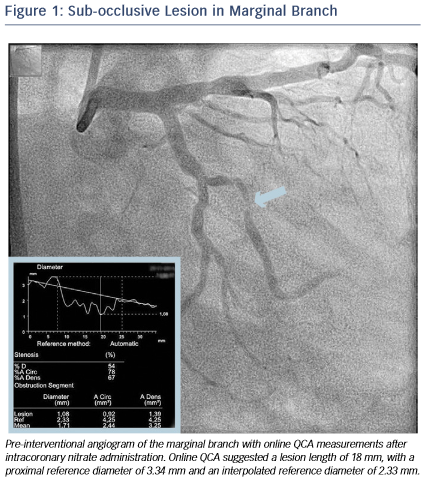
A 46-year-old male without cardiovascular history underwent coronary catheterisation for non-ST elevated myocardial infarction. Angiogram showed a sub-occlusive lesion in the marginal branch that was considered the culprit (see Figure 1), with an online measured interpolated reference diameter of 2.33 mm by Quantitative Coronary Angiography (QCA), while the lesion length was 18 mm.
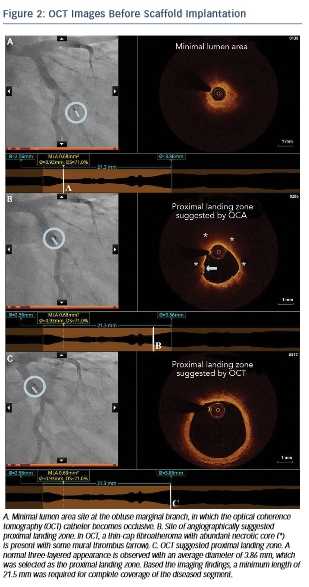
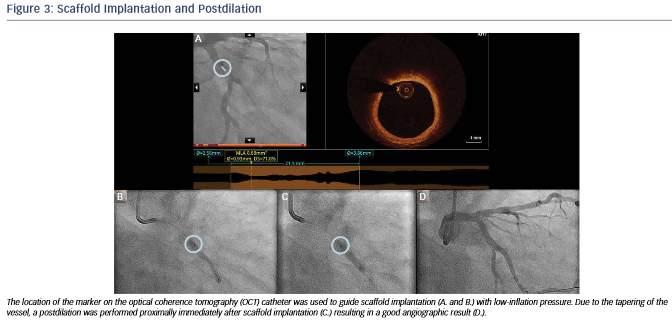
After predilation with a 2.5 x 15 mm balloon, an OCT pullback co-registered with the angiogram was acquired in order to assess the lesion, select device size and determine the landing zone. Cross-sectional OCT images (see Figure 2) revealed an occlusive lesion at the minimal lumen area. At the angiographically suggested proximal reference segment, OCT revealed the presence of a thin- cap fibroatheroma with mural thrombus. Therefore, another more proximal landing zone was selected based on the OCT images, with a mean diameter of 3.86 mm and a maximum diameter of 3.93 mm. As the maximum diameter in the proximal landing zone was below 4 mm, we selected a 3.5 mm AbsorbTM (Abbott Vascular, Santa Clara, CA, US) bioresorbable scaffold that can be safely expanded up to a 4 mm diameter. Also, seeing the high-risk plaque morphology at the angiography suggested landing zone, a 23 mm long scaffold was selected instead of the 18 mm suggested by angiography for complete coverage of the diseased segment. Furthermore, we decided upfront that postdilatation would be necessary in order to match the proximal reference diameter. A 3.5 x 23 mm scaffold was then implanted with low-pressure inflation in order to avoid vessel injury due to the tapering of the vessel distally.
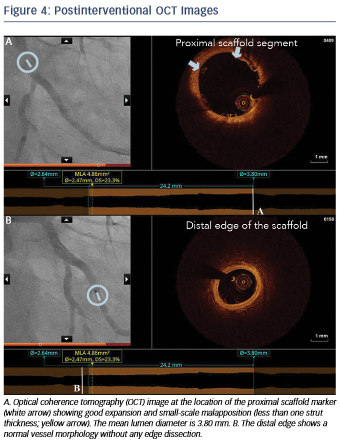
Immediately after implantation, a proximal postdilation was performed with a 3.75 x 15 mm noncompliant balloon (see Figure 3). A final OCT was performed to assess the implantation result, showing a good scaffold expansion with small-scale malapposition (less than one strut thickness) proximally that was accepted, and absence of vessel injury at the edges of the stent (see Figure 4).
Overall, in this case, OCT helped us to achieve optimal lesion coverage, select the size and length of the implanted scaffold that was different from what was suggested by online QCA and guide the use of proximal postdilation. Moreover, OCT helped confirm the absence of distal vessel injury and a good expansion and apposition.
Case study 2. Identification of Mechanism of Stent Thrombosis and Guidance of Treatment
A 68-year-old female had a history of primary percutaneous intervention of the Right Coronary Artery (RCA) two weeks earlier in another hospital with implantation of an UltimasterTM (Terumo Europe, Leuven, Belgium) 2.5 x 18 mm stent due to an acute inferior infarction. She presented in our centre with recurrent inferior ST-elevation myocardial infarction.
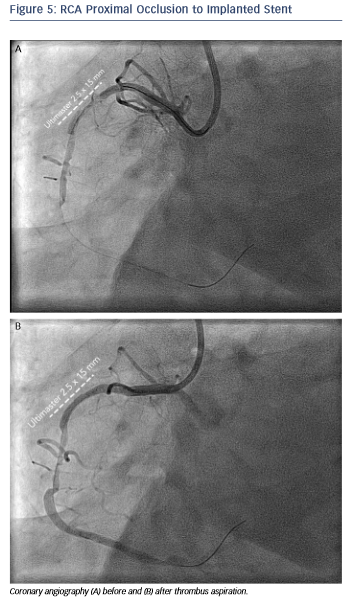
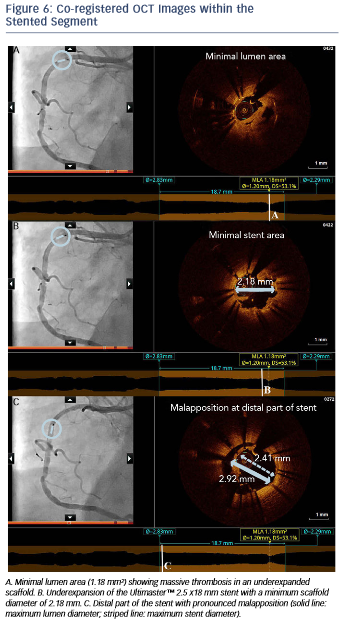
The angiogram revealed an occlusion of the RCA proximally to the previously implanted stent (see Figure 5). After thrombus aspiration, a co-registered OCT was performed to investigate the pathomechanism of the stent failure. Cross-sectional OCT images (see Figure 6) revealed the presence of an underexpanded and malapposed stent with thrombotic material at the site of the most severe underexpansion.
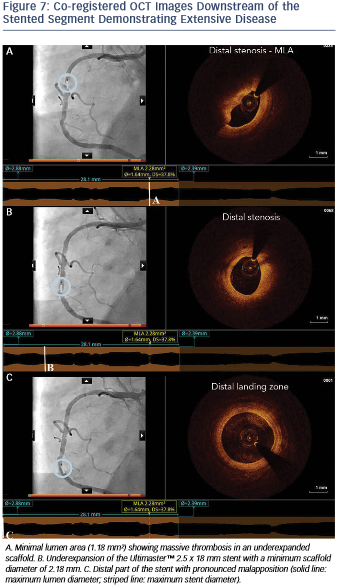
Moreover, OCT showed the presence of extensive disease distally to the stent comprising several stenotic lesions (see Figure 7).
Importantly, these findings of excessive malapposition, underexpansion and in-stent stenosis due to thrombus were not visible by angiography, while the severity of the downstream disease was also underestimated. A landing zone was selected based on the lumen profile view, aiming to cover the entire diseased segment. OCT measurements dictated the selection of a PromusTM 3.0 x 32 mm (Boston Scientific, Natick, MA, US), with the intention to distally overlap the pre-existing stent. Immediately postimplantation, after considering the lumen area at the site of the malapposition (2.67 mm) and a distal reference area of 2.82 mm, the balloon of the stent (3.0 mm diameter) was used for postdilation of the entire stented region, including the underexpanded and malapposed Ultimaster stent. This resulted in a well-expanded stent, landing in a relatively healthy segment and with a short segment of strut overlap (see Figure 8). The lumen area within the previous stent was also improved (Minimal Lumen Area (MLA) increased from 1.18 mm² to 5.29 mm²), as were apposition and expansion of this stent.
In this case, OCT helped us understand the pathomechanism of stent thrombosis and also revealed the presence of severe under-recognised atherosclerotic disease distally to the stent. The visualisation of the substrate together with the accurate measurements helped us selected the proper treatment, resulting in optimal lesion coverage and correction of the mechanical issues of the thrombosed stent.
Conclusions
OCT is an intravascular imaging modality with the potential to play an integral role in the daily cath lab routine. Information acquired by OCT is crucial in preprocedural planning, while OCT can be used to assess acute postprocedural result, guiding the performance or deferral of further intervention. Recently introduced technological developments providing a spatial co-registration of the OCT findings with the angiographic image can enable the operator to use this information for procedural guidance in an easy, quick and reliable manner. The adaptation of such imaging-guided strategies can aid decision-making in everyday practice while helping the optimisation of the procedural result.
Rx Only
Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.
Unless otherwise noted, TM indicates that the name is a trademark of, or licensed to, St. Jude Medical or one of its subsidiaries. ST. JUDE MEDICAL, the color gold, and the nine-squares symbol are trademarks and service marks of St. Jude Medical, Inc. and its related companies. © 2015 St. Jude Medical, Inc. All Rights Reserved.