Atrial Septal Defects and Patent Foramen Ovale
Nomenclature
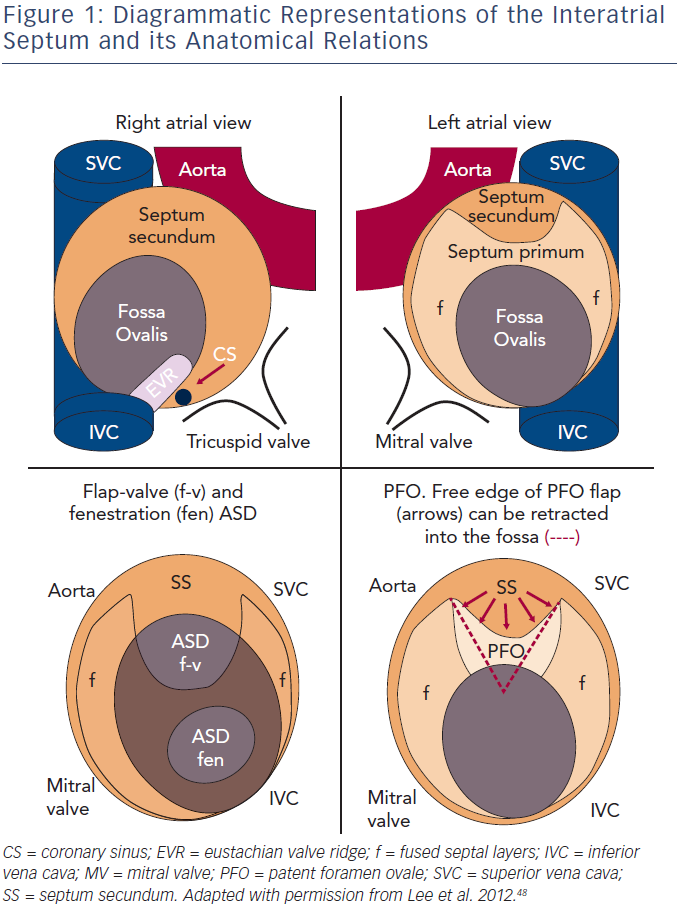
The atrial septum is a complex structure, with the true septum comprised of two layers containing a potential flap valve. The septum primum extends from caudal to cranial within the atria, on the left side of the septum secundum. The septum secundum is a crescent-shaped infolding of the atrial roof, extending from the anterosuperior aspects of the atria, and the hole left within the crescent is the fossa ovalis.1 The fossa ovalis is usually sealed shut by the (intact) septum primum (see Figure 1).
A fenestration-type secundum atrial septal defect (ASD) is formed by a defect, or fenestration, within the septum primum. However, a flaptype secundum ASD is also described, and is present when there is a deficiency in the septum primum, which fails to reach to the septum secundum. A patent foramen ovale (PFO) is characterised by the failure of the septum primum to fuse with the septum secundum after birth, and a probe-patent PFO occurs in approximately 25 % of the population.2
The distinction between a PFO and flap-type secundum ASD is not dichotomous but is on a spectrum, dependent upon the size of the gap remaining between the septum primum and secundum, which may change in size with cardiac loading conditions. However, it is generally accepted that an ASD, rather than a PFO, is present when the defect is present throughout the cardiac cycle.
Defects within the atrial septum may also occur in a variety of other positions. This review will focus primarily on those within the ‘true’ atrial septum (secundum ASD). Sinus venosus defects, coronary sinus defects and primum ASDs (partial atrioventricular septal defect [AVSD]) are beyond the scope of this article, but many of the pathophysiological and management principles still apply.
Current Management of Atrial Septal Defects and Patent Foramen Ovales
The consequences of left-to-right shunt across an ASD are right atrial (RA), right ventricular (RV) and to a lesser extent left atrial (LA) volume overload. This is associated with an increased pulmonary blood flow (raised Qp:Qs) and long-term repercussions that include right-sided heart failure and arrhythmias. The recommendations for medical or interventional treatments are summarised in the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines3 – closure is generally recommended unless the ASD is small (<5 mm) and asymptomatic with no evidence of cardiac compromise. However, even then progression of pathology should be closely monitored and age alone is not a contraindication to repair.4,5 The choice of surgical versus interventional device closure is based on ASD anatomical features and centre preference, but generally surgery is reserved for those defects with unsuitable anatomy.6
The management of PFOs is more controversial, and indications for closure are generally cryptogenic stroke or systemic arterial embolisation, with further considerations including decompression illness and possibly migraine with aura.2
Arrhythmias in Patients with Unrepaired Atrial Septal Defect and Patent Foramen Ovale
Prevalence of Arrhythmias
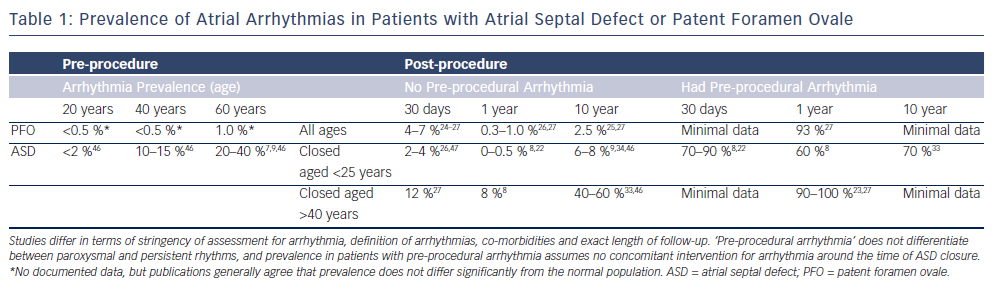
Atrial arrhythmias (AAs) – atrial fibrillation (AF) and/or flutter (AFL) – are significantly increased in patients with ASDs; the prevalences are summarised in Table 1. In unoperated adults, the estimated incidence of AAs is approximately 10 % under the age of 40 years,7 rising to at least 20 % with increased age, pulmonary arterial pressure and systemic hypertension.8,9 Additional studies have suggested that the incidence is increased in male patients, those with chronic obstructive pulmonary disease, reduced ejection fraction and hypertension.7 For patients with a PFO, the incidence of AA prior to defect closure does not appear to be significantly raised.2 However, the evidence is limited and is confounded by the fact that many PFOs are only detected following thromboembolic events, which may represent undetected episodes of AA in some cases.
To our knowledge there is no conclusive evidence of an increased risk of ventricular arrhythmias in patients with ASDs, despite the evidence of geometric and electrical remodelling of the RV myocardium.10 There are reports of sudden cardiac death in patients with both repaired and unrepaired ASD, but much of the risk could represent coronary artery disease in an older population.11
Pathophysiology of Arrhythmias
The left-to-right shunt enabled by the presence of an ASD results in cardiac remodelling secondary to long-standing haemodynamic overload. It is this geometrical remodelling that plays an important role in the pathogenesis of AAs. Changes in atrial tissue properties, including interstitial fibrosis, increased myocyte size and alterations in ultracellular structure have been described12,13 and predispose to AF in particular.14
Electrical remodelling has also been widely described, even in the absence of AA. The P-wave duration and dispersion is increased,15,16 with lengthened sinus node recovery time and conduction delay across the crista terminalis,15 which is likely to play a key role in the arrhythmogenesis of AFL. Interestingly, in contrast to studies of conventional patients with AF, the atrial effective refractory period (AERP) is usually lengthened in the ASD patients.15
The majority of geometrical remodelling is reported on the right side of the heart, which is subject to the most dramatic flow and pressure changes. However, there are also left-sided geometrical changes in those with an ASD, with moderately increased LA dimensions found on cardiac magnetic resonance (CMR)17,18 and electroanatomical mapping (EAM).13 Importantly, these changes are also associated with electrical remodelling. Roberts-Thomson et al. compared a group of patients undergoing ASD closure with those with left-sided accessory pathways, and again found prolonged AERP and slower conduction in those with ASDs, alongside reduced bipolar voltage and enhanced inducibility of AF.13 Finally, the possibility of abnormalities of the native interatrial conduction pathways, secondary to the anatomical defect, should also be considered.
In summary, remodelling of both the right and left atrium contributes to arrhythmogenesis and the heterogeneity of that remodelling process between atria may contribute to the propensity for native arrhythmias in this patient group.
Management of Pre-procedural Atrial Arrhythmias
Standard pharmacological management strategies for arrhythmias should be employed in patients with ASDs and arrhythmias, with an aim to restore sinus rhythm where possible. AF should be treated with both antiarrhythmic therapy and anticoagulation,3 and direct-current (DC) cardioversion has a similar safety and efficacy profile to that performed in patients without structural heart disease.19 However, the presence of AAs should be considered an indication for closure of an ASD, unless there is a compelling reason not to do so. Although the rhythm may not revert to sinus post-procedure, there are likely to be benefits in terms of symptoms.4
Arrhythmias After Atrial Septal Defect Closure
Reverse Remodelling Post-closure
Following ASD closure, there is a rapid decrease in RV and RA volumes, but this improvement is not universal.17,18,20 Cardiac geometric reverse remodelling is reduced in older patients and those who have developed markers of decompensation, such as higher pulmonary arterial pressure (PAP) and larger right-sided chambers.20 In particular, those with AF pre-procedure are less likely to improve, and there is even evidence that the LA volumes actually increase post-procedure in those with AF.18
The evidence of electrical reverse remodelling post-closure is much more limited than that for geometrical reverse remodelling. In a small follow-up study, Morton et al. found that there were no significant changes in AERP, sinus node recovery time or transcristal conduction, but some trends towards improvement.15 There are also more generalised changes found on the surface electrocardiogram (ECG), with reduction of P-wave duration, P-wave dispersion and QT dispersion.10,21
The comparison between device and surgical closure is skewed by the inherent difference in substrate, with surgical closure now generally reserved for larger ASDs. Once patient selection is controlled for, a significant difference in arrhythmia prevalence post-closure has not been demonstrated, despite the presence of an atriotomy and sutured patch7 in the surgical group.
Patients with Pre-procedural Arrhythmias
There is strongly supportive evidence that the prevalence of AAs is decreased following closure of an ASD. On meta-analysis of the available literature, the odds ratio for AA prevalence immediately post-procedure (<30 days) is 0.80 (95 % confidence interval [CI] 0.66–0.97), improving to 0.47 (95 % CI 0.36–0.62)) over mid-term follow-up (30 days to five years).7,22 Up to 40 % of patients with pre-procedural arrhythmias revert to sinus rhythm8,21 (see Table 1).
However, many AAs may recur in the longer term (>5 years), their progression stalled rather than terminated. Younger patients and patients with paroxysmal (rather than permanent) AF or AFL prior to ASD closure are more likely to remain in sinus rhythm.8,22 In contrast, although other studies have demonstrated no change in rhythm for the elderly and those with permanent AF at the time of device closure,4,23 there is clear symptomatic improvement in terms of ventricular function and markers of cardiac failure.
The longest term outcome (>10 years) is less clear-cut. Attie et al. randomised patients over 40 years old to surgical or medical treatment of ASD, and they were followed up for a mean of seven years. At enrolment, 21 % had AF or AFL, with a further 5 % noted to have another supraventricular tachycardia. Over the course of follow-up, a further 8 % developed AAs, with no significant difference between the interventional and non-interventional groups.9
Patients without Pre-procedural Arrhythmias
Pathophysiology of New-onset Arrhythmias
The majority of long-term post-procedural arrhythmias are likely to be related to progression of pre-existing atrial substrate abnormalities, with an increased incidence with age and severity of markers of remodelling secondary to the ASD. However, there are subsets of arrhythmias that do not conform to this paradigm.
The first comprises patients with very early onset arrhythmias, in the days and weeks following closure. For those undergoing device closure, it has been hypothesised that the early, transient arrhythmias are likely to be triggered by local irritation. This applies particularly to the PFO closure patients who are unlikely to have suffered from the same extent of atrial remodelling pre-closure, with minimal intracardiac shunt. Incidences range from 1 to 6 %, but arrhythmias will cease in up to two-thirds of these patients by one-year post-procedure.22,24–28 Whilst there is no evidence of a device-specific effect in the ASD closure group, there is suggestion that some PFO closure devices may entail a slightly higher risk.25
The second group are those with new right-sided arrhythmias following surgical closure. Right-sided arrhythmia circuits may involve the atriotomy scar, the cavotricuspid isthmus (CTI), the patch site or combinations of these in multi-loop circuits. A dual-loop reentry circuit involving reentry around both the tricuspid annulus (CTI-dependent) and the atriotomy scar is not uncommon.29 In addition, areas of abnormal conduction related to scar may serve not only as a crucial pathway of slow conduction in the formation of reentrant circuits, but also as the site of origin of a focal atrial tachycardia.29–31 Surgical technique for closure of ASDs has also evolved with increased employment of the anterolateral minithoracotomy, subxiphoid and limited median sternotomy approaches, and their implications for the atriotomy should be considered in the electrophysiological approach to these patients.
The third group comprises those patients who have undergone device closure and develop persistent arrhythmias related to the closure device itself. It is worth noting that a potential central barrier of non-conduction (the defect) for formation of a reentrant circuit pre-exists any closure device, but a native reentrant atrial tachycardia around an ASD is a rare event. However, there remains the possibility of device-related inflammation and scarring, with subsequent anisotropic conduction. Such a mechanism is rare, but there are reports of macroreentrant circuits occurring de novo post-procedurally around the device itself,32 although device type or size does not affect incidence.27 Finally, it has been hypothesised that the interatrial conduction, largely via Bachmann’s bundle lying in the roof of the atrium at the superior margin of the fossa ovalis, may be compromised by surgical or device closure. This is reflected in the finding of increased P-wave duration index postclosure,26 and may provide further substrate for arrhythmogenesis.
Long-term Risk of Arrhythmias Post-closure
Over the longer term, it appears that the risk of new AAs is increased in proportion to the extent of pre-closure exposure to haemodynamic remodelling forces. In the longest follow-up studies of the surgical population, freedom from arrhythmia is good for those whose ASD was repaired at a young age and prior to development of raised PAPs (up to 97 % arrhythmia free at 27 years).33
On the other hand, the risk of AAs is much higher for older patients, especially those undergoing closure over the age of 40 years. In the two years following ASD or PFO closure, de novo AF has been reported in 12 % and 7 %, respectively, well above the baseline population risk.8,27 Late after closure (>20 years), an AA prevalence of up to 59 % has been found in the oldest patients.33
There are also findings of long-term sinus node dysfunction, with a prevalence of up to 40 % in long-term surgical follow-up studies.34 This is consistent with the findings of pre- and post-procedural sinus node dysfunction on electrophysiological (EP) study.15
Management of Post-procedural Arrhythmias
Interventional Techniques
It is clear that the incidence of AF is raised in the ASD population post-closure, and left atrial access can pose considerable problems. In the surgical population, conventional needle puncture positioning techniques are hampered by the loss of the second ‘jump’ of the puncture needle into the fossa ovalis. In addition, the patch or native septum may be tougher (especially Gore-Tex® patches), thickened or fibrotic and balloon dilation of the puncture site may be needed prior to passage of the transseptal sheath.35 Radiofrequency-assisted transseptal puncture may offer advantages over conventional transseptal techniques.36
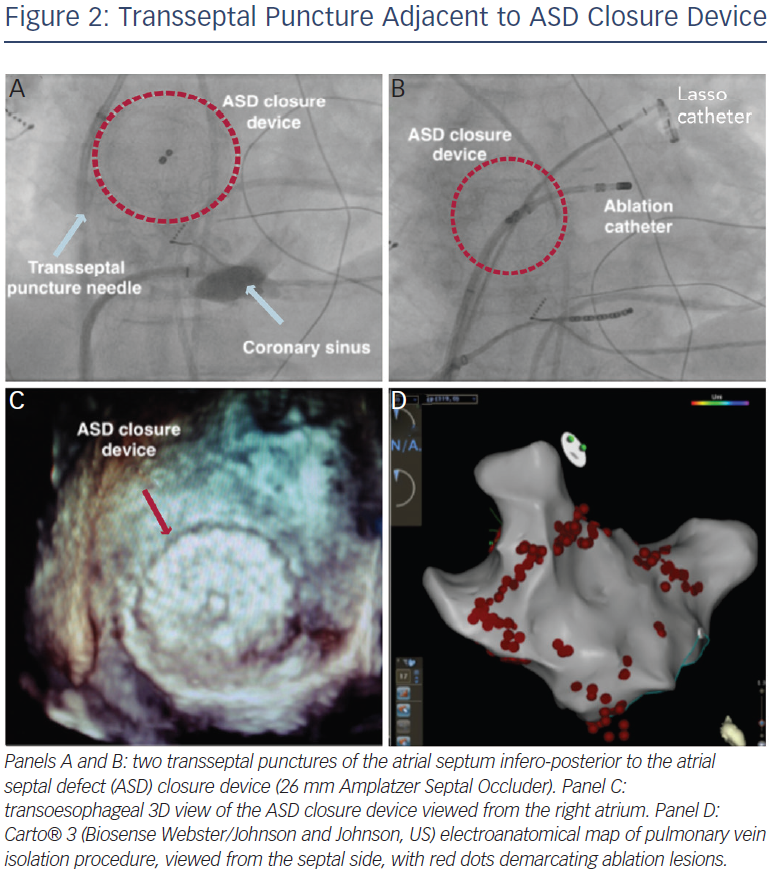
The presence of a closure device provides an obstacle to transseptal access. There are multiple methods for circumventing this issue – it is often possible to find a portion of atrial septum not covered by device, and transoesophageal echocardiography or intracardiac echocardiography (ICE) are invaluable in this regard.35,37–39 Pre-procedural computed tomography (CT) may also assist in puncture site selection.39 A suitable puncture site, once identified, is most often infero-posterior (see Figure 2). Radiofrequency (RF) energy-assisted septal perforation can be considered,37 but there are ex vivo reports that suggest that caution is necessary when RF energy is used in close proximity to a device. Pedersen et al. tested a RF transseptal needle (Bayliss Medical Company Inc, Montreal, Canada) with an Amplatzer device in a saline bath, and arcing and damage to the device was noted with direct contact of the needle.39
For those patients where the whole of the true septum is covered, typically with devices >26 mm in diameter, there are further options. The ASD device may be clearly visualised on fluoroscopy or ICE, and a Brockenbrough transseptal needle may be used to enter the LA through the device itself. However, most devices may be resistant to the advancement of the sheath, and high pressure balloons (generally angioplasty balloons) or multiple sequential dilations may be used to enable access.37,38,40 Alternatively, a retrograde approach via the aorta using a magnetic navigation system has also been described.41
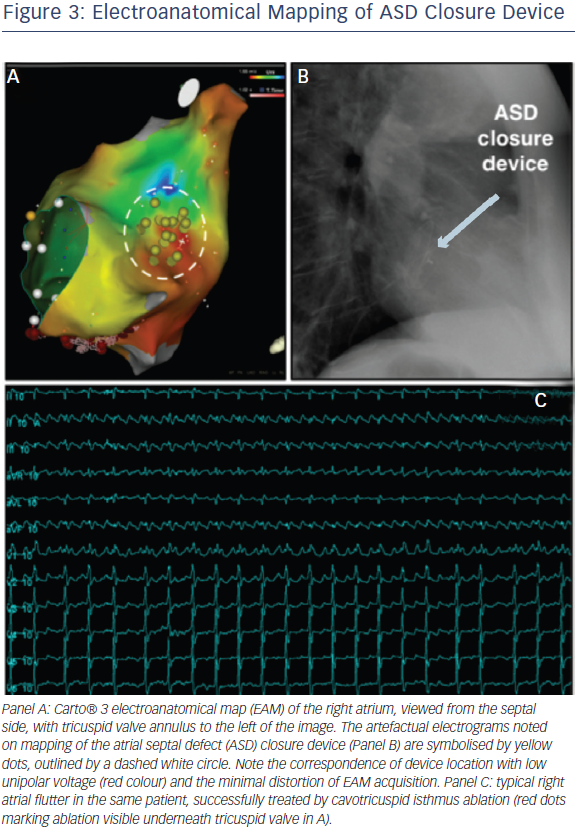
Once access has been established, conventional LA mapping and ablation techniques are used similar to those employed in the normal adult population, with no evidence of any device impact on EAM function (see Figure 3, EAM generated anatomy of the right atrium, with no significant impact of device presence). The atrial arrhythmia substrate remains incompletely understood and many of the recent developments in mechanistic mapping of AF42 have not yet been translated to the adult congenital heart disease (ACHD) population, largely because of the relatively smaller numbers of eligible patients. There is an increased incidence of atrial fibrosis in ASD patients manifesting as regions of low voltage amplitude and spontaneous electrical scarring. This is accompanied by regional atrial conduction delay, double potentials, fractionated potentials and increased inducibility of sustained AF.13
Overall, reported success rates following pulmonary vein isolation (PVI) are close to those achieved in patients without ASD.35,37 Concerns of post-procedural residual shunt and displacement of the device have so far proved to be unfounded, but most operators have advocated waiting at least six months after device placement prior to electrophysiological intervention in the event that a pre-closure procedure has not been performed.37,40
Right-sided arrhythmias are also more common in the ASD population than controls, and standard techniques can be employed for their management. It should be noted that non-CTI dependent macroreentry circuits are often present, particularly in the surgical population. For these patients, consideration should be given to ablating from the atriotomy to the inferior vena cava (IVC), in addition to a CTI line, even when the only identified circuit is CTI dependent (see Figure 3).29 Focal atrial tachycardias and arrhythmias common in all patient groups, such as atrioventricular (AV) nodal reentry tachycardias, may also occur.
Pre-emptive Treatment
One approach that has been initially successful has been to combine an interventional closure of the ASD with DC cardioversion, which may be effective in restoring and maintaining sinus rhythm in at least the medium term.22 However, intermittent episodes of paroxysmal atrial fibrillation (PAF) were noted prior to reversion to long-standing sinus rhythm, and it has been suggested that cardioversion might be best postponed for 3–6 months, once atrial reverse remodelling has occurred.
The idea of combining ASD closure with an arrhythmia intervention is not a new one, and surgical closure has previously been combined with a maze-type procedure4,43 or irrigated RF ablation44 with good results. There are also very limited reports of RF catheter ablation prior to device closure of ASD at a separate procedure.45
Conclusions
ASDs are associated with an increased prevalence of atrial arrhythmias. Timely closure of a significant ASD results in a reduced incidence of future arrhythmias, and may also reduce arrhythmia burden in patients who have already developed an arrhythmia prior to closure. However, patients remain at an increased risk of AAs in the long term compared with the normal population, with the greatest increase in risk in older patients or those with evidence of haemodynamic complications related to the ASD.
Given the future propensity for AAs, and the challenges of intervention post-device closure, there may be a subgroup of patients that would benefit from risk stratification and prophylactic interventional electrophysiological procedures, a dilemma that as yet remains unanswered. Alternatively, increased adoption of biodegradable devices and new interventional EP techniques may help alleviate some of the issues related to interventional EP procedures in this group.