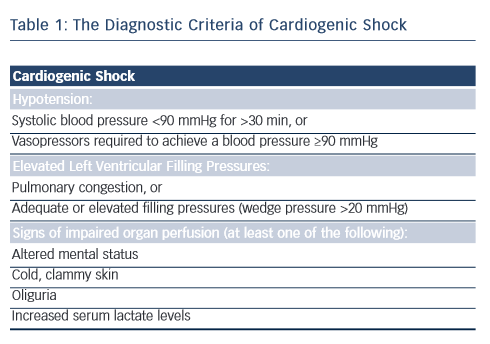
Cardiogenic shock is a clinical condition of inadequate end-organ perfusion due to cardiac dysfunction (see Table 1). It most commonly occurs in the setting of acute MI with left ventricular failure (~80 % cases),1,2 but can also be caused by right ventricular infarction or late mechanical complications, such as acute mitral regurgitation or ventricular rupture (septal or free wall). Non-infarct-related cardiogenic shock is comparatively rare, and may result from decompensated valvular heart disease and arrhythmias, to name a few mechanisms.
The pathophysiology of cardiogenic shock is complex. Myocardial ischaemia induces marked depression of myocardial contractility, this sets into motion a downward spiral of reduced cardiac output and hypotension, which in turn drives further myocardial ischaemia. This severe cardiac dysfunction causes tissue hypoperfusion and may eventually result in death if the vicious cycle is not adequately interrupted by timely treatment measures. In addition to the physiological impairment of myocardial function, cardiogenic shock also induces deleterious systemic responses including pathological vasodilation (after compensatory vasoconstriction), systemic inflammation with capillary leakage and impairment of the microcirculation.1,3 This review will look at the optimal management of patients with cardiogenic shock complicating acute MI, with particular focus on revascularisation therapy and the use of mechanical circulatory support devices.
Incidence and Prognosis of Cardiogenic Shock
Cardiogenic shock complicates 5–10 % of acute MI cases, and despite advances in acute care there remains the same incidence (~60,000– 70,000 patients per year in Europe).2,4
Historically, MI complicated by cardiogenic shock was associated with a mortality rate of 80–90 %.5 However, with advances in coronary reperfusion techniques over the past few decades, especially with the introduction of primary percutaneous coronary intervention (PCI), the mortality rate has improved to below 50 %.4,6–12 The trend towards better outcomes may also be due to greater awareness of the need for timely treatment, improvements in the medical care of haemodynamically unstable patients as well as the use of mechanical support devices, although this has not yet been clearly demonstrated.
Despite this high mortality rate, it is important to note that patients with cardiogenic shock who survive to discharge have a long-term outcome similar to that of patients without cardiogenic shock, with a good functional outcome at 1 year.13,14 This highlights the importance of improving the chance of early survival among patients in cardiogenic shock.
Management
Myocardial Reperfusion
There is evidence that the high mortality rates associated with cardiogenic shock have improved over time.7,9,11,15,16 This benefit is thought to be due to increased use of coronary revascularisation strategies, which, by restoring flow to the ischaemic myocardium, can limit infarct size as well as interrupt the downward spiral that characterises cardiogenic shock.7,9,15
As such, the cornerstone of the management of cardiogenic shock complicating acute MI is prompt revascularisation, as highlighted in the Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial.17 Patients with cardiogenic shock were randomly assigned to initial medical stabilisation or early revascularisation (PCI or coronary artery bypass grafting [CABG] within 6 hours of randomisation and 18 hours of onset of shock). The primary endpoint (all-cause mortality at 30 days) did not differ between the initial medical stabilisation and early revascularisation treatment groups; however, there was a significant decrease in mortality rates at 3 and 6 years in patients assigned to early revascularisation.14,17,18 The number needed to save one life at 1 year by early revascularisation in comparison with initial medical stabilisation is less than eight, and this benefit remained with long-term follow-up.
The SHOCK trial also demonstrated the importance of timely revascularisation, with increasing long-term mortality rate as time to revascularisation increased from 0 to 8 hours. However, the overall benefit of revascularisation in cardiogenic shock may extend past the traditional 12-hour window to potentially 54 hours post-MI and 18 hours after the onset of shock.18,19
In current European Society of Cardiology (ESC) guidelines, early revascularisation by either PCI or CABG for cardiogenic shock is recommended,20 but despite a general increase in the trend to perform early revascularisation, real-world rates remain relatively low (50–70 %).2,14,21
Anti-platelet and Anti-thrombotic Medication
The clinical syndrome of cardiogenic shock impairs enteral absorption, which may result in suboptimal bioavailability of oral agents.22 In addition, patients in cardiogenic shock often require mechanical ventilation and this poses problems with oral medication (often overcome with nasogastric tube insertion and delivery of crushed tablets), which further complicates matters.23 In general, patients with cardiogenic shock should be given aspirin as is routinely recommended in acute coronary syndromes; however, administration of oral P2Y12 inhibitors should be deferred until coronary angiography, as CABG may be immediately required.20 Although not yet licensed in the UK, cangrelor (a fast-acting and rapidly reversible intravenous P2Y12 inhibitor) may prove to useful in these situations where oral anti-platelet administration may be delayed or unreliable.24
Given the abovementioned problems with oral administration of anti-platelet agents, glycoprotein IIb/IIIa (GP IIb/IIIa) inhibitors may be beneficial in the management of cardiogenic shock. Observational data suggest a potential mortality benefit with their use in treating cardiogenic shock, but one randomised trial (of only 80 patients) did not demonstrate any benefit of routine abciximab administration compared with use at the discretion of the interventional practitioner.25 As such, current guidelines recommend use of GP IIb/IIIa inhibitors as bailout therapy for thrombotic complications during PCI and limiting their routine use during PCI for ST-segment elevation MI (STEMI).20,26
During PCI, adjunctive anticoagulation with unfractionated heparin, low-molecular-weight heparin or direct thrombin inhibitors should be co-administered with anti-platelet therapy. With a lack of specific randomised trials in cardiogenic shock, the same recommendations apply as for other types of acute coronary syndromes.20
What is the Ideal Method of Early Revascularisation?
Coronary reperfusion can be achieved with thrombolytic therapy (in patients with STEMI), PCI or emergency CABG. There is a paucity of randomised data assessing the efficacy of thrombolytic therapy compared with either placebo or PCI in patients who have cardiogenic shock at presentation. Studies have demonstrated some benefit of thrombolytic therapy compared with placebo, but superiority of PCI or CABG over thrombolytic therapy.18,27,28 Therefore, thrombolytic therapy is recommended only if PCI is not possible or if it is delayed (>90 min) and presenting early after symptom onset (<3 hours), followed by emergent transfer to a PCI facility.20
The prognosis of patients with cardiogenic shock is related to the procedural success of PCI and importantly, patients with cardiogenic shock are less likely to have successful PCI than patients without shock.16 Since the recruitment for the SHOCK trial (where only 37 % of patients undergoing PCI received stents) there have been many advances in PCI: first, bare-metal stents and more recently drugeluting stents have been associated with a greater likelihood of complete revascularisation, a higher incidence of Thrombolysis In MI-3 flow and improved survival rates in patient in cardiogenic shock.29–31
In the current European guidelines, infarct-related cardiogenic shock is an indication for emergency revascularisation with either PCI or CABG, if the patient has suitable coronary anatomy.26 To date, there exist no randomised clinical trials comparing PCI with CABG in patients with cardiogenic shock. In the SHOCK trial, the study protocol recommended CABG for patients with a left main coronary stenosis of ≥50 %, ≥2 total or subtotal occlusions, stenosis of >90 % in two non-infarct-related major arteries or stenosis unsuitable for PCI, as well as in patients whose PCI was unsuccessful.17 However, this decision was made on an individual basis by site investigators and PCI was often performed in patients with three-vessel disease. Among the 128 patients with cardiogenic shock receiving emergency revascularisation (63 % PCI and 37 % CABG) there was a similar mortality rate at 30 days, 1 year and 6 years regardless of the method of revascularisation.14,17,18 However, in current practice, few patients with cardiogenic shock and three-vessel disease are referred for CABG, ranging from 3.2 % to 8.8 %,32 possibly reflecting the real-world difficulties of arranging emergency CABG for patients who often present with cardiogenic shock overnight and at weekends.
In summary, in patients with cardiogenic shock complicating acute MI, PCI allows prompt restoration of coronary flow, which may arrest the vicious cycle of myocardial ischaemia and reduced cardiac output. If there is likely to be a significant delay to PCI, thrombolytic therapy should be considered. Finally, urgent CABG should also be considered in the case of unsuccessful PCI, left main disease, three-vessel disease or in the presence of severe valvular disease and mechanical complications of MI.20,26
Revascularisation of Multi-vessel Coronary Artery Disease
The majority (70–80 %) of patients with cardiogenic shock complicating acute MI have multi-vessel disease, which in itself is associated with a higher mortality rate than single-vessel disease.4,33–35 As discussed above, the current evidence does not clearly identify an optimal revascularisation strategy for patients with cardiogenic shock with multi-vessel disease. There are four observational reports comparing PCI with CABG that suggest similar mortality rates;36 however, in current practice, CABG is rarely performed in patients with cardiogenic shock.2,33
Due to the lack of reliable prospective clinical data, guideline recommendations have been based on physiological principles to arrest the downward spiral of myocardial ischaemia and reduced cardiac output. In contrast to the recommendations for haemodynamically stable patients, current guidelines recommend PCI to the culprit lesion followed by PCI to critical lesions (>90 % stenosis) or those with unstable appearances (possible thrombus or lesion disruption) if there is on-going ischaemia or haemodynamic instability.20,26 The on-going prospective, multicentre CULPRIT-SHOCK trial will company culprit-vessel treatment with complete revascularisation in patients with cardiogenic shock.
Revascularisation of Left Main Stem Disease
There are no current guidelines on revascularisation for patients with left main coronary artery (LMCA)-related MI complicated with cardiogenic shock. In recent years, together with the increased use of PCI for LMCA in the stable setting, PCI has become the preferred method of revascularisation for patients with LMCA-related acute coronary syndromes.37 The combined SHOCK trial and registry only include 21 patients with LMCA-related MI and there is significant treatment bias in favour of PCI (as many severely unstable patients will be unsuitable for surgical revascularisation), as such it is not possible to draw any valid conclusion from their outcomes.14,38
Given the paucity of evidence, the decision to perform CABG or PCI in patients with cardiogenic shock and LMCA disease should be made on an individual basis taking into account the clinical stability of the patient, coronary anatomy, operator experience and potential risks of either strategy.20,26
Pharmacological Management
There have been recent summaries on the use of inotropes and vasopressor agents in patients with cardiogenic shock,39,40 and a review of this is beyond the scope of this article. In brief, regardless of the decision to revascularise, pharmacological stabilisation of the patient in cardiogenic shock is a complex process that requires judicious use of fluids to obtain euvolaemia, vasopressors and inotropes with the aim of preventing multi-organ hypoperfusion and ultimately failure. Despite their almost ubiquitous use and clear effect on haemodynamics, there are no randomised data showing a prognostic benefit with the use of inotropes or vasopressors in the setting of cardiogenic shock. Furthermore, as catecholeamines increase myocardial oxygen consumption and vasoconstrictors may impair the microcirculation as well as tissue perfusion, their use should be restricted to the lowest possible dose for the shortest possible duration.
Mechanical Circulatory Support
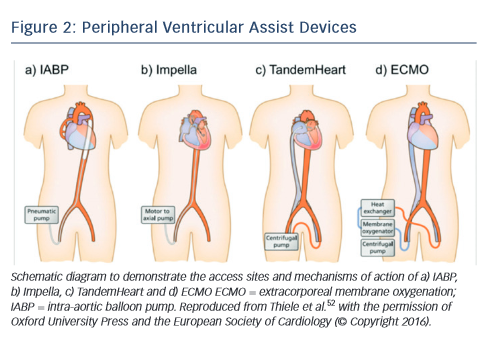
Mechanical circulatory support should be instituted in patients with cardiogenic shock who remain haemodynamically unstable despite revascularisation and inotrope therapy.41,42 In general, mechanical circulatory support devices can potentially be of benefit in cardiogenic shock by maintaining organ perfusion while reducing myocardial oxygen demand and augmenting coronary blood flow. Historically, the intra-aortic balloon pump has been the only mechanical circulatory support device available to interventional practitioner during high risk PCI such as with a patient in cardiogenic shock.33 More recently, a number of new devices have become available, including axial flow pumps (e.g. Impella), left atrial to femoral artery bypass pumps (e.g. TandemHeart®) and new devices for the implementation of extracorporeal membrane oxygenation (ECMO) (see Figure 2).
Intra-aortic Balloon Pump
The intra-aortic balloon pump (IABP) remains the most commonly used form of circulatory support in patients with cardiogenic shock. The IABP has two major components: a balloon catheter (filled with helium) and a pump console to control the balloon (see Figure 2a). It is commonly inserted via the femoral artery, and the balloon inflates with the onset of diastole (around the middle of the T-wave) and deflates at the onset of left ventricular systole (at the peak of the R-wave).41 This mechanism provides haemodynamic support by increasing diastolic perfusion pressure in the coronary arteries and reducing left ventricular afterload, thereby reducing wall tension and myocardial oxygen demand, resulting in a modest elevation in cardiac output (0.3–0.5 l/min).
The first randomised controlled trial comparing IABP therapy with conservative management in patients with cardiogenic shock (IABPSHOCK; n=45) found a reduction in BNP, but no change in clinical outcomes.43 This was followed with a larger trial of 600 patients with acute MI complicated by cardiogenic shock and randomised patients to either IABP or standard therapy (IABP-SHOCK II), which did not demonstrate a significant reduction in mortality rate at 30 days or 12 months (although 86.6 % of IABPs were inserted post-PCI).44 Current ESC guidelines advise against the routine use of IABP during PCI in patients with cardiogenic shock, and limit their recommendations of its use to patients with cardiogenic shock due mechanical complications of MI who are awaiting surgery.20,26
Left Atrial to Aorta Assist Devices
The TandemHeart is a percutaneously inserted circulatory assist device that pumps blood extracorporeally from the left atrium to the iliofemoral arterial system via a transeptally placed atrial cannula, bypassing the left ventricle (see Figure 2b).41 By working in parallel with the left ventricle, this results in a reduction of left ventricle preload, filling pressures, wall stress and myocardial oxygen demand while increasing arterial blood pressure and systemic perfusion (increasing cardiac output up to 4 l/min).
A retrospective analysis of patients with refractory cardiogenic shock demonstrated that the TandemHeart improved haemodynamics.45 This was following by two small randomised controlled trials that demonstrated improved haemodynamics with TandemHeart compared with IABP, but at a cost of increased complications such as severe bleeding, limb ischaemia and arrhythmias.46,47
Left Ventricle to Aorta Assist Device
The Impella is a non-pulsatile axial flow Archimedes-screw pump designed to propel blood from the left ventricle into the ascending aorta in conjunction with the left ventricle (see Figure 2c).41 This results in direct unloading of the left ventricle, an increase in forward flow associated with reduction in myocardial oxygen consumption, improvement in mean arterial pressure and reduction in pulmonary capillary wedge pressure. A number of different versions are available: the percutaneous 12-F (Impella 2.5) device and 21-F (Impella 5.0) surgical cut-down device, which provide maximal flow rates of 2.5 and 5.0 l/min, respectively. More recently, a percutaneous 14-F (Impella CP®) device provides an intermediate level of support similar to the TandemHeart (up to 4 l/min). Complications of Impella support include bleeding at the vascular access site, haemolysis and pericardial tamponade, whereas use is contraindicated in patients with severe peripheral vascular disease, presence of a mechanical aortic valve or a severely calcified aortic valve.
There have been a number of studies demonstrating the safety and haemodynamic benefits of Impella insertion in patients with cardiogenic shock. Recently, in the Efficacy Study of Left Ventricular Assist Device to Treat Patients with Cardiogenic Shock (ISAR-SHOCK) trial, the Impella 2.5 was associated with a greater increase in cardiac output and mean arterial pressure compared with IABP; however, the there was no difference in mortality rates between the two groups.48
Extracorporeal membrane oxygenation
The most comprehensive percutaneously inserted mechanical support is provided by ECMO, which can either provide oxygenation only (veno-veno ECMO) or oxygenation with circulatory support (venoarterial [V-A] ECMO). In cases of biventricular failure, V-A ECMO is the mechanical circulatory support device of choice and is able to provide 7 l/min of non-pulsatile flow.41 Similar to cardiopulmonary bypass circuits, V-A ECMO involves a circuit composed of a centrifugal pump, a heat exchanger and a membrane oxygenator. A venous cannula (20- F) drains blood from the right atrium into a membrane oxygenator for gas exchange, and then oxygenated blood is pumped into the patient via an arterial cannula (17-F) (see Figure 2d).
The main limitation of ECMO is that the retrograde flow of the peripheral arterial cannulation increases afterload, increasing myocardial oxygen demand and can precipitate pulmonary oedema. Conversely, increasing ECMO flow rates in this situation will worsen the haemodynamic situation. A number of techniques can be used to improve left ventricular emptying, including concurrent Impella usage, or venting with a pigtail catheter in the left ventricle, or creation of an atrial septal defect. Failing resolution, central ECMO can be used with direct cannulation of the left ventricle, left artery or pulmonary artery. There are non-randomised data using historical controls suggesting that ECMO use for patients with MI-related cardiogenic shock can improve survival rates.49–51 Although promising, using historical controls rather than a prospective randomised study does not account for other potential temporal advances in management.
Although ECMO may improve survival of patients in cardiogenic shock, there is significant procedural morbidity; common complications include limb ischaemia, renal failure, bleeding and infection.41
Conclusion
Early revascularisation remains the cornerstone of the management of patients with cardiogenic shock, although the optimal method remains unclear; patients who have the earliest revascularisation have the best outcomes. In addition to restoring myocardial perfusion, management of patients with cardiogenic shock requires haemodynamic stabilisation, predominantly through careful use of vasopressors and inotropes, which may increase myocardial oxygen demand and thereby cause worsening ischaemia. In more recent years, a number of mechanical circulatory support devices have emerged that provide promising adjuvant therapies for patients in cardiogenic shock. These will allow for angioplasty to be performed in an improved haemodynamic setting and provide a bridge to potential recovery.