Heart failure (HF) can be defined haemodynamically as any abnormality of cardiac structure or function resulting in a failure to deliver oxygen at a rate adequate for tissue requirements, despite normal filling pressures – or only at the expense of increased filling pressures.1 Around half of patients with HF have reduced left ventricle ejection fraction (LVEF; EF <40 %) at rest (HF-REF).2
Diagnosis of suspected HF starts with medical history, physical examination, 12-lead electrocardiogram, chest X-ray and natriuretic peptide measurement. Transthoracic echocardiography is the first-line imaging modality.3–5 Cardiovascular magnetic resonance (CMR) imaging plays an important complementary role in evaluating the underlying aetiology (or aetiologies) of the suspected HF, informing prognosis and guiding decision making, particularly where echocardiographic windows are inadequate or findings are inconclusive. CMR is not part of the assessment process in acute HF owing to reduced monitoring capability, patient intolerance of lying flat and reduced image quality (arrhythmias and reduced ability to breath hold). For the diagnosis of the ambulatory patient with suspected HF, CMR receives a class IC recommendation in the European Society of Cardiology (ESC) HF Guidelines.1 CMR is frequently used in the management of HF patients: the European CMR registry reported that the most common indications for CMR include risk stratification in suspected ischaemia, assessment of viability and assessment of suspected myocarditis and cardiomyopathy.6
The image signal in MR arises from hydrogen nuclei, which are aligned to the field of the scanner and then ‘excited’ by radiofrequency wave pulses. Energy is released as the excited nuclei or spins relax back to equilibrium magnetisation. Decay of the longitudinal and transverse components of magnetisation are exponential processes named T1 and T2 relaxation, respectively. Whereas T2 relaxation takes into account dephasing due to random proton–proton interaction, T2* relaxation is a faster process, as it also takes into account dephasing accelerated by local inhomogeneities in the global magnetic field. Datasets in patients with HF typically start with T1-weighted blackblood spin-echo sequences for anatomy, gradient-echo (bright-blood steady-state-free precession) cine sequences in three long-axis and three short-axis planes to acquire chamber volumes, and contrast-enhanced inversion-recovery gradient-echo sequences with appropriate nulling of normal myocardium to look for late gadolinium enhancement (LGE). Further tissue characterisation sequences are acquired selectively to answer specific questions.
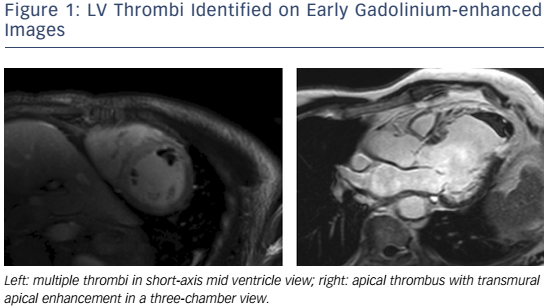
The advantages of CMR over other non-invasive imaging modalities are accuracy, reproducibility,7,8 unrestricted field of view, lack of ionizing radiation, and the ability to characterize myocardial tissue. CMR is the gold standard modality for the assessment of LV volumes and EF,9,10 LV thrombus (see Figure 1),11–13 left atrium (LA) volumes14 and the right ventricle (RV).15 CMR tissue characterisation techniques may include inversion recovery images acquired either early (for thrombus imaging) or late (for scar imaging) after contrast administration, diffuse fibrosis assessment with T1 mapping and extracellular volume (ECV) measurement,16–18 iron concentration using T2* and non-contrast ‘native’ T1 measurements, oedema evaluation using T2-weighted images, fatty infiltration with fat saturation sequences or T1-weighting, perfusion imaging with first-pass T1-weighted images and metabolism assessment using MR spectroscopy (MRS).19 Its limitations are its availability, cost, the exclusion of patients with non-MR-compatible devices,20 cerebrovascular clips or metallic objects in the eye, and the inability to scan patients who are too breathless to lie flat or who have claustrophobia. In patients with HF, electrocardiographic gating may be challenging in those with AF, high ectopic burden or broad QRS width. Furthermore, linear gadolinium chelates are contraindicated in individuals with estimated creatinine clearances <30ml/min, and renal dysfunction is relatively common in HF. Newer macrocyclic chelates have a better safety profile, but should still be used with caution in patients with advanced renal dysfunction.21
Ischaemic Cardiomyopathy
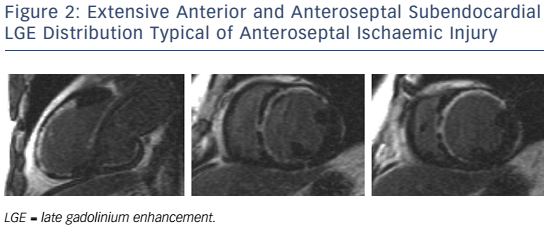
In patients presenting with de novo acute HF and no clinical or electrocardiographic suggestion of ischaemic aetiology, LGE-CMR is sensitive and specific for the presence of underlying significant coronary artery disease (CAD).22,23 Identifying ischaemic cardiomyopathy (ICM) as the aetiology of HF implies a worse prognosis than non-ICM.24 Patients with single-vessel disease (<75 % luminal stenosis) not involving the proximal left anterior descending (LAD) or left main arteries and with no history of MI or prior revascularisation have a prognosis similar to patients with non-ischaemic HF.25 However, the absence of angina and significant stenoses on coronary angiography does not exclude CAD as the cause of HF, as infarction may follow coronary spasm or embolism, or be followed by coronary recanalisation.26 Around 15 % of patients with unobstructed coronaries are found to have LGE in distributions typical of prior infarction and would be misclassified as having dilated cardiomyopathy (DCM) were LGE-CMR imaging not performed.27,28 Patterns typical of prior infarction show subendocardial or transmural enhancement respecting one or more coronary territories, reflecting the ‘wavefront phenomenon’ of ischaemic injury (see Figure 2).29
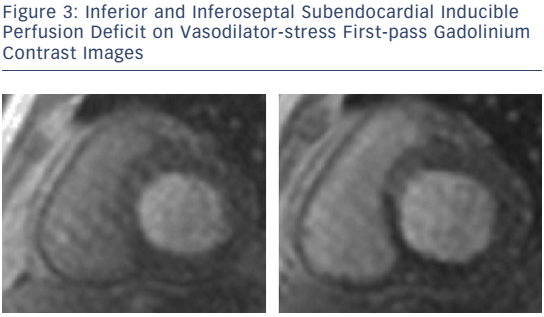
For the detection of suspected stable CAD in patients with intermediate (15–85 %) pre-test probability of disease and preserved and reduced LVEF, vasodilator stress CMR for first-pass perfusion and dobutamine stress CMR for inducible wall-motion abnormalities (WMA) are well established, feasible and safe (see Figure 3).30–33 Quantitating deformation with strain-encoded CMR improves the accuracy of high-dose dobutamine stress CMR over visual assessment of WMA on cine imaging.34,35 Threedimensional stress perfusion techniques acquire datasets covering the whole heart rather than three short-axis slices, allowing quantitation of ischaemic burden with good agreement with stress perfusion singlephoton emission computed tomography (SPECT).36,37 High-resolution stress perfusion CMR is feasible in patients with HF.38
In hearts with resting LVEF ≤40 % and established left-main, leftmain-equivalent or significant proximal LAD and multivessel disease (with fractional flow reserve <0.80), coronary revascularisation is indicated for the relief of angina and for ‘prognosis’ (ESC/European Association for Cardio-Thoracic Surgery class IA recommendation).39 CMR strategies for estimating the likelihood of improvement include assessing the response to low-dose dobutamine, extent of LGE transmurality, and myocardial thickness.40 CMR myocardial feature tracking reduces inter-observer variability compared with visual analysis of the response to low-dose dobutamine.41 Contractile reserve correlates inversely with infarct transmurality, but cannot be straightforwardly predicted in segments with infarction of intermediate transmural extent.42 Greater transmurality of infarction as assessed by LGE-CMR has been shown to correlate inversely with the likelihood of segmental and global functional recovery post revascularisation.43–45 LGE transmurality is often used as a surrogate for viability, the attraction being that no stress or metabolic imaging step is required, and in a meta-analysis of prospective trials was shown to carry the highest sensitivity and negative predictive value for recovery.46
Multiple earlier reports and a meta-analysis showed that the presence of viability in patients with ICM, as assessed by thallium nuclear perfusion SPECT, 18-F fluorodeoxyglucose positon emission tomography or dobutamine echocardiography, predicted improved survival after revascularisation.47–50 However, the viability substudy of the Surgical Treatment for Ischemic Heart Failure (STICH) trial did not find an association between viability and outcomes on multivariate analysis.51,52 However, this study was criticised for the following reasons: the protocol was amended to make viability testing optional and at the investigators’ choice performed by either SPECT or dobutamine echocardiography, the definition of viability was not prospectively validated and did not require segments to be hypocontractile at rest, and post-revascularisation regional and global changes in LV function were not reported.53 Further trials investigating the predictive value of CMR viability are warranted.
A meta-analysis of studies of subjects with known or suspected CAD showed that the presence and extent of LGE predict future major adverse cardiovascular events (MACE) and mortality;54 however, many of these studies did not recruit subjects with HF. In a large cohort with mostly preserved EF, LGE and WMA inducible upon dobutamine stress were independent predictors of MACE; conversely, absence of inducible WMA predicted excellent prognosis over the following 3 years.55,56 However, in one study in which patients with LVEF <55 % and regional WMA at rest were recruited, dobutamine-induced increases in wall-motion score index provided additive predictive power for MACE beyond resting LVEF when resting LVEF was >40 %.57 In studies of patients with ICM and reduced EF, greater extents of scar volume as a proportion of total myocardial volume independently predicted MACE,58,59 and larger peri-infarct ‘intermediate zones’ independently predicted mortality60,61 and inducibility of monomorphic ventricular tachycardia.62
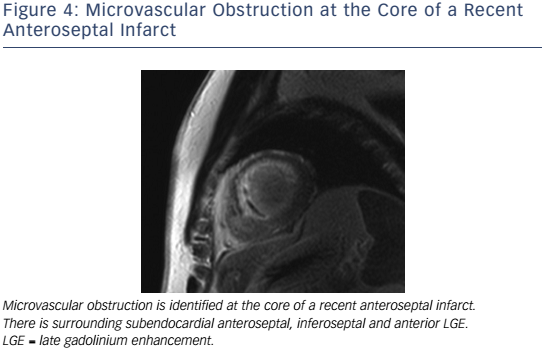
In the setting of recent acute MI, CMR correlates of higher risk include LVEF, infarct and peri-infarct zone sizes, the presence of microvascular obstruction (MVO) (see Figure 4), reduced RVEF,63 and lower degrees of myocardial salvage, assessed as the difference between the area at risk on T2 weighting and the final infarct size.64 CMRdetected MVO, defined as a lack of gadolinium retention (dark region) in the core of a segment surrounded by tissue showing gadolinium enhancement, is an independent predictor of MACE65,66 and adverse LV remodelling.67 Greater extents of late MVO (assessed 15 minutes after gadolinium administration), rather than early MVO (1 minute after), independently predict MACE after primary percutaneous coronary intervention for ST-segment elevation MI.68 Hypointense infarct cores on T2 weighting, a marker of intramyocardial haemorrhage (IMH), are associated with larger infarcts and greater extents of late MVO, and predict MACE and adverse LV remodelling independent of the presence of MVO.69 IMH, alternatively detected by a hypointense infarct core with T2* <20 ms, also independently predicts MACE and adverse LV remodelling.70
Dilated Cardiomyopathy
Dilated cardiomyopathy (DCM) is a clinical diagnosis based on dilation and systolic dysfunction of the left or both ventricles that is unexplained by abnormal loading conditions or CAD.71 CMR studies have shown that increased native T1, LA volumes and RV dysfunction, but not greater degrees of trabeculation, are independent predictors of survival and HF outcomes in patients with DCM.72–75
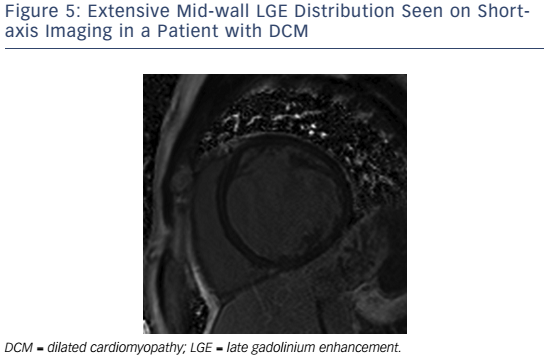
If present in DCM, LGE is typically found in a mid-wall distribution (see Figure 5).27,76 Co-existent endocardial LGE may indicate concurrent ischaemic contribution to HF aetiology. Mid-wall LGE was found in 10–28 %28,27 of patients with DCM in adult case series, but may be less common in children.77 In adults, the presence of LGE in non-ischaemic DCM independently predicts an increased risk of MACE, including hospitalisation for decompensated HF, sudden and non-sudden cardiac death, ventricular arrhythmia78,79 and all cause mortality.80 Mid-wall LGE distribution confers a higher risk of inducible ventricular tachycardia.81 Larger extents of mid-wall LGE independently predict lower likelihoods of LV reverse remodelling in patients with recent-onset DCM.82
Cardiac energy metabolism is deranged in patients with HF, and MRS is the most powerful method for its non-invasive assessment in vivo.83 Its clinical use has been limited owing to its low temporal and spatial resolution, but this is arguably less important in diffuse myocardial processes such as DCM. In DCM, myocardial phosphocreatine:adenosine triphosphate ratios are reduced and lower ratios independently predict mortality.84 Forward creatine kinase shuttle flux is reduced in non-ischaemic cardiomyopathy and this also independently predicts mortality.85,86 The hypothesis that altered energetics play a causal role in HF remains controversial and the modulation of substrate use as a therapeutic target remains under investigation.87
Takotsubo Syndrome
Takotsubo syndrome is an acute and usually reversible HF syndrome whose presentation mimics an acute coronary syndrome (ACS).88,89 Recently proposed diagnostic criteria include transient and reversible regional WMA of the LV or RV frequently preceded by a stressful trigger, circumferential involvement of ventricular segments beyond a single coronary territory, the absence of culprit coronary events and viral myocarditis, new and reversible electrocardiographic changes, significant increases in natriuretic peptide levels, and troponin level increases that are modest for the degree of dysfunction.90 CMR detects ‘typical’ apical ballooning and ‘atypical’ variants (e.g. biventricular, midventricular, basal and focal ballooning).91–93 Oedema is detected on T2-weighted CMR in both takotsubo and myocarditis. However, LGE is usually absent acutely in takotsubo,94,95 unlike in MI (subendocardial) and acute myocarditis (non-ischaemic distribution). Where available, CMR is recommended within 7 days of presentation in suspected takotsubo syndrome to aid diagnosis and detect LV thrombus, and to confirm myocardial recovery on follow-up.96
Myocarditis
Myocarditis is an inflammation of myocardial tissue of infectious, immune or toxic aetiology that presents as an ACS, new-onset or worsening HF or life-threatening arrhythmia in the absence of CAD or known causes of HF. It may resolve spontaneously, recur or become chronic, and may predate the development of DCM. Strictly speaking, myocarditis is diagnosed when endomyocardial biopsy (EMB) findings meet certain histological, immunohistochemical and immunological criteria.97,98 In life-threatening presentations, urgent EMB has a class 1B indication, as only EMB can distinguish aetiologies (e.g. viral from nonviral, lymphocytic from giant-cell). However, as EMB may be limited by sampling error or complicated by tamponade, it is not recommended for all patients.99 CMR is the primary non-invasive imaging modality for the assessment of suspected myocarditis in clinically stable patients – it supports the diagnosis by identifying abnormalities of cardiac structure, function and tissue characteristics; excludes ischaemic patterns of injury; and acts as a gatekeeper to EMB.100,101
The accuracy of CMR diagnosis of myocarditis is variably reported, and depends on the combination of techniques used, the time point in the inflammatory process at which images are taken, the severity of the inflammation in the group studied and whether EMB is the comparison standard. Combining tissue characterisation techniques improves diagnostic performance.102 The recommended clinical diagnostic algorithm for suspected myocarditis98 includes the CMR criteria proposed in the 2009 Journal of the American College of Cardiology White Paper for CMR assessment of myocarditis.103
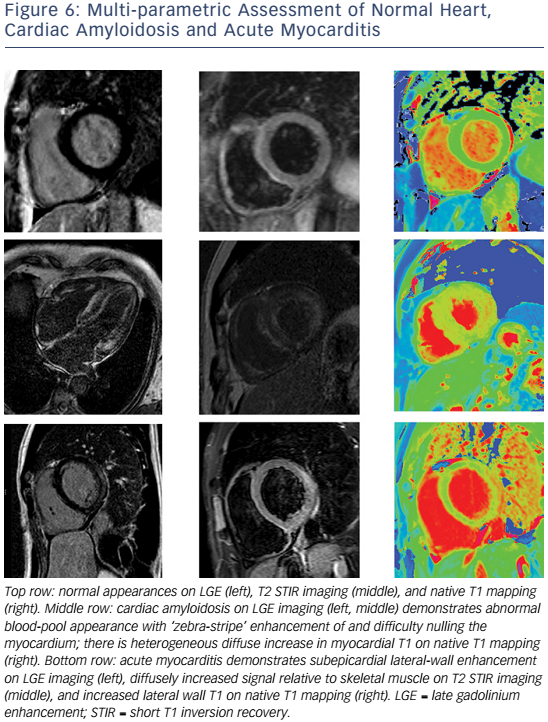
These recommendations require two of the following three criteria for diagnosis: T2-weighted images showing increased global or regional myocardial signal intensity relative to skeletal muscle (indicating myocardial oedema), early gadolinium-enhanced T1-weighted images showing increased global myocardial signal intensity relative to skeletal muscle (indicating myocardial hyperaemia/capillary leak) and ≥1 focal lesion on LGE with non-ischaemic distribution (indicating necrosis/fibrosis). The most common LGE distribution is focal and patchy and involves the subepicardial lateral wall.104,105
The sensitivity of the 2009 CMR criteria is greatest for patients with infarct-like, rather than HF, presentations.106 Where conventional techniques do not detect abnormalities or where gadolinium is contraindicated, native (non-contrast) T1 mapping (see Figure 6) can detect oedema in non-ischaemic distributions and thus improve diagnostic confidence when imaging is performed at a median of 3 days from presentation.107,108 Native T1 values raised >5 standard deviations above the mean of the normal range independently identified acute myocarditis and were more raised in acute compared with convalescent stages of the process.109 Conversely, in another study of patients with recent-onset HF and clinically suspected myocarditis, T2 mapping revealed higher median global myocardial T2 values in those with biopsy-proven active myocarditis, while there were no significant differences in native or post-contrast global myocardial T1.110
The prognostic value of CMR findings in myocarditis requires further investigation. The presence of LGE on CMR within 5 days of presentation was an independent predictor of all-cause and cardiac mortality in patients with biopsy-proven viral myocarditis,111 and in an LGE-positive cohort, initial LVEF, but not LGE extent, predicted outcome.112
Iron Overload Cardiomyopathy
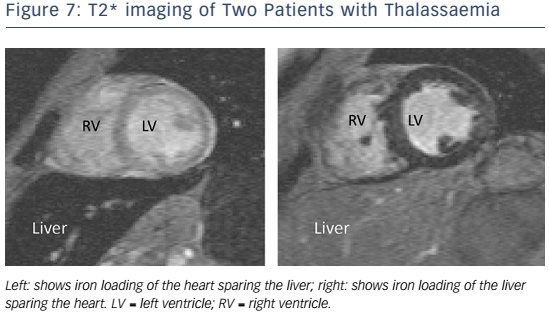
In patients with HF and suspected cardiac iron overload, and especially in those with transfusion-dependent beta-thalassaemia major, CMR with T2* at 1.5 T field strength should be performed at the earliest opportunity to expedite definitive diagnosis and treatment, and advice from a centre of expertise should be sought.113 T2* is a magnetic relaxation property of any tissue and is inversely related to intracellular iron stores. Myocardial T2* <20 ms is a reproducible, specific marker of significant cardiac iron content, which does not correlate with liver iron or serum ferritin concentrations (see Figure 7).114 Myocardial T2* and iron concentration in the septum are excellent predictors of mean total cardiac iron concentration in explanted hearts.115 T2* declines before LVEF, and is the best predictor of future HF and ventricular arrhythmias, with T2* <10 ms indicating high risk and 10–20 ms indicating intermediate risk.116 If iron chelation therapy is started early, declines in LVEF are preventable and reversible; T2* imaging has had a major impact on survival in patients with thalassaemia.117
Cardiac Amyloidosis
Amyloidosis results from extracellular deposition of abnormal insoluble fibrils derived from a misfolded, normally soluble protein.118 The three most common types of amyloidosis affecting the heart include systemic amyloid light-chain (AL) amyloidosis, where the fibrils derive from monoclonal immunoglobulin light chains in the setting of B-cell dyscrasias, hereditary systemic (variant) TTR amyloidosis, where the fibrils derive from variant transthyretin, and senile systemic (wild-type) ATTR amyloidosis. The V122I variant is the most common mutation and is found in 3–4 % of African Americans, carriers of which have an increased risk of HF compared with non-carriers over long-term follow-up.119 ATTR amyloidosis is an underdiagnosed cause of HF. Biopsy remains the gold standard for diagnosis.120
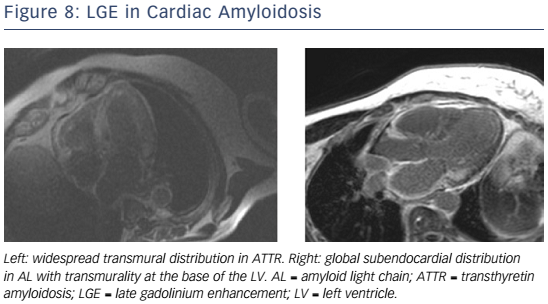
Given the short median survival in cardiac AL amyloidosis (∼5 months), CMR is indicated in patients with HF and suspected cardiac amyloidosis to expedite diagnosis and treatment with chemotherapy. CMR findings in cardiac amyloidosis reflect interstitial expansion with high myocardial gadolinium uptake, and typically reveal global subendocardial or transmural LGE, shortening of subendocardial T1, rapid blood pool wash-out and suboptimal myocardial nulling (see Figure 8).121–124
Compared with AL, ATTR involvement is characterised by greater LV mass and LGE extent, greater likelihood of transmural and RV LGE, and longer survival.125 Elevated T1 on native T1 mapping (see Figure 6) has high accuracy for cardiac involvement in AL amyloidosis126 and together with raised ECV predicts mortality.127 Raised native T1 also has high accuracy in ATTR cardiac amyloidosis as compared with HCM, ATTR mutation carriers and normal controls, and may represent an early disease marker.128 The value of native T1 as a marker of disease burden during therapy is under investigation in international trials of TTR-specific therapies.
Anderson–Fabry Disease
Anderson–Fabry disease (AFD) is an X-linked recessive disorder caused by reduced or absent activity of the enzyme alpha-galactosidase A, resulting in lysosomal glycosphingolipid accumulation in several organs. LV hypertrophy (LVH), fibrosis, HF (initially with preserved EF) and sudden arrhythmic death may occur.129,130 In the presence of renal replacement therapy, cardiac involvement drives mortality. Early enzyme replacement therapy can cause regression of LVH.131
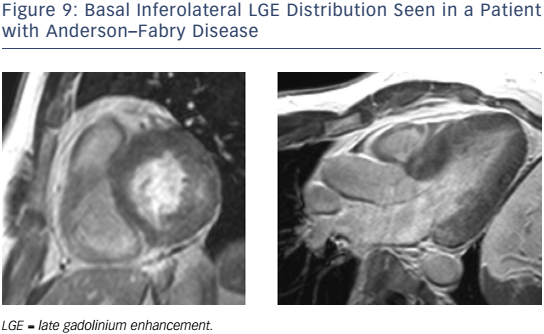
In patients with AFD, LGE may be detected particularly affecting the basal inferolateral wall in the absence of CAD (see Figure 9).132,133 Native myocardial T1 is reduced in AFD,134,135 differentiating this condition from HCM, oedema and amyloidosis, where T1 is increased. CMR can identify AFD in unexplained LVH and offers the potential for early detection. In one study, native T1 was lowered in patients with genotype-positive, LVH-negative AFD, although correlation with lipid burden on biopsy was not performed.136 Current guidelines advocate the CMR measures of LV wall thickness, mass index and LGE to guide enzyme replacement therapy in patients with AFD.137
Heart Failure with Preserved Ejection Fraction
The diagnosis of HF with preserved EF (HF-PEF) requires the following criteria: resting LVEF ≥50 %; a non-dilated LV (indexed volume <97/ml/m2); and sufficient biomarker, imaging and/or invasive evidence of diastolic dysfunction.138 Although outcomes are similar in patients with HF-PEF and HF-REF,139 no drug therapies have been shown to improve survival in HF-PEF to date.140,141
While 2D-echocardiography has superior temporal resolution for assessment of LV filling, CMR may contribute superior assessment of LVEF, LV mass and LA volumes, in addition to correlates of pulmonary hypertension (e.g. pulmonary artery:aorta ratio142 and RV function143). The use of correlations between diastolic dysfunction and diffuse myocardial fibrosis144 and between post-contrast T1 and outcome145 is under investigation and CMR indices of diastolic function are not yet routinely measured.146–148
Cardio-oncology
Detection and management of the cardiotoxic effects of anticancer treatments is of growing importance.149,150 Treatments implicated in causing LV dysfunction include anthracyclines, cyclophosphamide, docetaxel, bortezomib, trastuzumab, bevacizumab and sunitinib.151,152 Most children with cancer will become long-term survivors and be more likely to develop HF than their siblings.153 Early detection and prompt treatment of anthracycline-related cardiotoxicity can prevent LV dysfunction and promote LV recovery.154–156
The Trastuzumab Trials Cardiac Review and Evaluation Committee defined treatment-related cardiac dysfunction as a symptomatic fall in LVEF by >5 % to <55 %, or an asymptomatic fall in LVEF by >10 % to <55 %.157 Current criteria for discontinuing trastuzumab depend on detection of LVEF 40–49 % and ≥10 % below baseline or LVEF <40 %.158 Compared with radionuclide cardiac blood pool imaging and echocardiography, the ‘standard’ imaging modality for serial LVEF assessment – CMR – offers a radiation-free, more accurate modality for detecting LVEF <50 %.159 Expert consensus guidelines recommend CMR in particular when ventricular function nears thresholds for chemotherapy discontinuation or when there is significant regurgitant valve disease.160
LGE is an insensitive marker with poor prognostic utility in cancer survivors.161 The use of ECV, LA volume, oedema, and deformation imaging to detect cardiotoxicity before declines in LVEF is under investigation.162–166 In a series of childhood cancer survivors who previously received ≥200 mg/m2 anthracycline and had normal indices of global systolic function by standard CMR parameters, CMR tagging techniques detected significant falls in global and segmental LV peak longitudinal and circumferential strain and detected more widespread regional falls in strain than did speckletracking by echocardiography.167
Other Cardiomyopathies
HF is an uncommon first presentation for HCM, arrhythmogenic RV cardiomyopathy, and cardiac sarcoidosis, and the role of CMR in the assessment of these conditions has been reviewed extensively elsewhere.26,168–171
Conclusion
CMR has established and evolving roles in the assessment of patients with HF, particularly the confirmation of underlying aetiology. The extent of involvement detected on CMR carries prognostic information in patients with ICM, DCM, iron overload, cardiac amyloidosis and AFD. The identification of diffuse interstitial fibrosis in many of the cardiomyopathies is increasing knowledge about the mechanisms of the disease processes involved. The role of T2* in assessment of response to therapy in iron overload is established and a potential role for T1 in specific therapies for cardiac amyloidosis and AFD is emerging. The use of T1, T2 and T2* mapping sequences is increasing for myocardial tissue assessment in the cardiomyopathies.