Chronic heart failure (HF), a complex and heterogeneous clinical syndrome, is a major cause of morbidity and mortality worldwide, and represents a major challenge to health care systems. The prevalence of HF and the number of hospitalisations is rising, even more in the ageing population.1 The direct costs of HF management reached 1–2 % of total health care expenditure and approximately two-thirds are attributable to hospitalisations. In 2012, in 197 countries, covering 99 % of the world’s population, the overall cost of HF management was estimated at US$108 billion per annum, and is predicted to rise.2
HF is caused by structural and/or functional cardiac abnormality, mainly by following aetiologies: coronary artery disease, arterial hypertension, valvular heart disease, inflammatory heart disease and/or primary cardiomyopathy. The typical symptoms are breathlessness, ankle swelling and fatigue, and may be accompanied by signs such as elevated jugular venous pressure, pulmonary crackles and peripheral oedema.3
Based on assessment of left ventricular ejection fraction (LVEF) HF is classified as HF with reduced LVEF (HFrEF; EF <40 %) and HF with preserved LVEF (HFpEF; EF >50 %). If LVEF is in the range of 40–49 % (‘grey area’), it is defined as HF with mid-ranged LVEF (HFmrEF).4 Around 60 % of total HF patients has HFrEF, which is associated with high reninangiotensin- aldosterone and sympathetic nervous systems activation. Most recent therapeutic improvements in these patients are due to the use of pharmacological agents that modulate these neuro-hormonal axes, angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), beta-blockers and mineralocorticoid receptor antagonists (MRAs). The beneficial effects of these treatments include the reduction of HF mortality by approximately one-third in a period of two decades. Despite this success, HF mortality rates remain high: the 5-year survival is worse than in many cancers.5,6
The 2016 European Society of Cardiology (ESC) HF guidelines recommend ACEI and beta-blockers as first-line therapy in symptomatic patients with HFrEF.4 However, both registries and clinical reports underline that the treatment of HF patients is suboptimal much more than expected, and that the heart rate is increased despite beta-blocker therapy.7–12 The registry of 12,440 patients demonstrated heterogeneity in treatment strategies, due to the drug side effects and contraindications.7 A European registry13 reported that only 17 % of patients were receiving the optimal combination and recommended dose of diuretic, ACEI and beta-blockers. Results from a French registry on 50,000 HF patients14 also confirmed suboptimal treatment of HF, demonstrating that after the first month following hospitalisation for worsening HF, only 47 % received an ACEI, 54 % a beta-blocker, and 17 % MRAs. The I Brazilian Registry of Heart Failure (BREATHE)15, conducted in 57 hospitals in Brazil, revealed that 69 % of HF patients were receiving an ACEI or ARB, 60 % a beta-blocker, and 49 % MRAs. However, only 17 % were receiving all three drugs together. Therefore, a need to identify a novel pathways and strategies for HF treatment is obvious and clinically justified. This article reviews the clinical evidence and guidelines recommendations for the use of two novel therapies in HF: ivabradine and trimetazidine.
Use of Ivabradine in Heart Failure
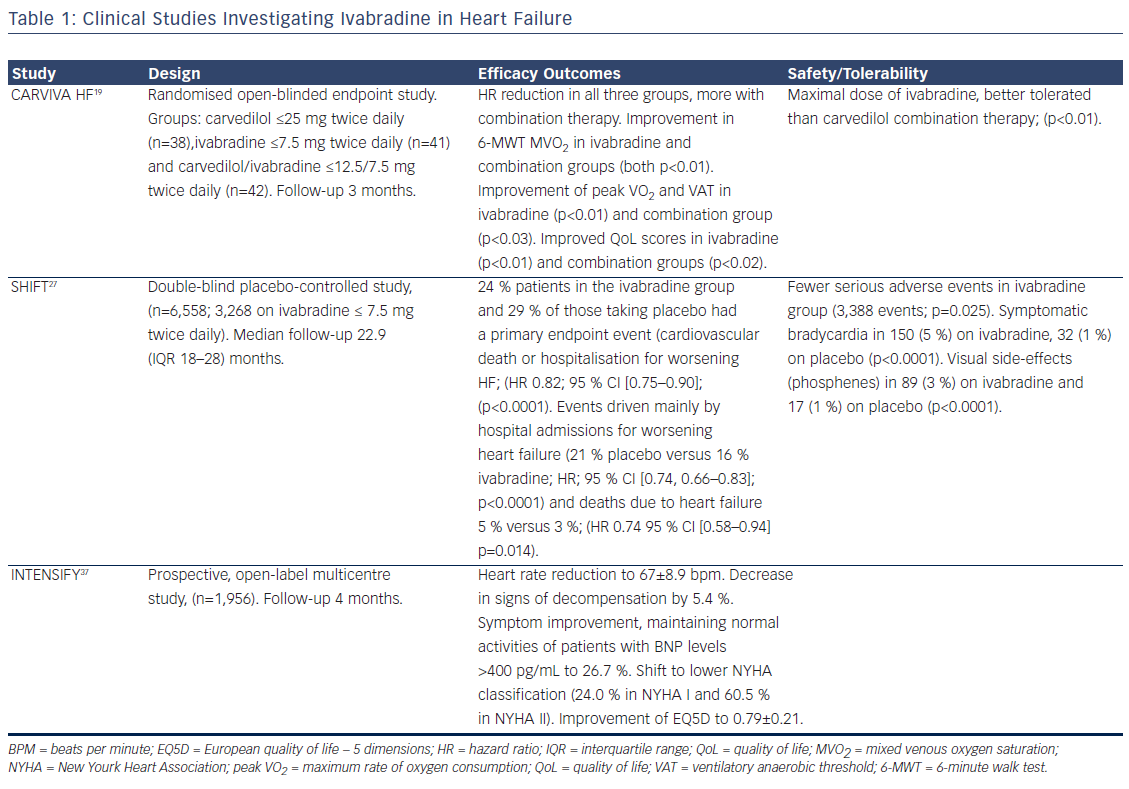
The mechanism of ivabradine’s beneficial effect is based on heart rate reduction at rest and/or exercise, which prolongs diastolic perfusion time, improves coronary blood flow, and increases exercise capacity. In contrast to beta-blockers, ivabradine causes increase in stroke volume, which may have beneficial cardiac effects. In both preclinical and clinical studies, ivabradine exerted an anti-remodelling effect, improving left ventricle (LV) structures and functions.16 In patients with HF, ivabradine not only reduces heart rate but also improves heart rate variability. No significant bradycardia, ventricular arrhythmias or supraventricular arrhythmias were reported.17 The efficacy and safety of ivabradine in HF has been established by two pivotal clinical trials and an open-label study (see Table 1). The effect of the drug can be assessed regarding four clinical aspects: functional improvement and symptoms relief, amelioration of the uality of life (QoL), effects on rehospitalisation and mortality and safety profile in HF.
Functional Improvement and Symptoms Relief
The evidence supporting functional improvement in patients with HFrEF is convincing. Treatment with ivabradine 7.5 mg twice daily resulted in improvement of functional parameters and exercise capacity. In addition, it was correlated with a significant increase in LVEF and a reduction in N-terminal pro-brain natriuretic peptide (NT-proBNP). The selection of the patients represented not only difficult treatment challenges but also the real-daily clinical practice pattern. It should be noted that use of ivabradine significantly improves the exercise capacity, gas exchange, functional HF class, QoL and neurohormonal modulation in patients with ischaemic HF.18 Furthermore, the CARVedilol, IVAbradine or their combination on exercise capacity in patients with Heart Failure (CARVIVA-HF) trial found that ivabradine alone or in combination with carvedilol was more effective than carvedilol alone in improving exercise tolerance and QoL in HF patients.19 If a patient is treated concomitantly with betablocker and ivabradine, the smaller doses and briefer titration period can be noticed. The addition of ivabradine to carvedilol in 69 patients in sinus rhythm, ischaemic HF (NYHA class II–III) and HR ≥70 beats per minute (bpm) revealed a shorter beta-blocker up-titration period, higher final beta-blocker dose as well as greater heart rate reduction and better exercise capacity.20
Amelioration of the Quality of Life
Improvement in symptoms and maintaining normal activities are important treatment goals for patients with chronic disabling diseases like HF. In the first weeks of ivabradine treatment, dyspnea at rest, exercise capacity and fatigue were improved. In a sub-analysis of the SHIFT trial (Systolic Heart failure treatment with the If inhibitor ivabradine Trial) in 1944 patients, health-related quality of life, as recorded by the disease-specific Kansas City Cardiomyopathy Questionnaire, was found to be inversely associated with clinical events.21 Treatment with ivabradine was associated with improvements in QoL scores and better outcomes. That was due to the improvement in exercise capacity and symptoms. The reported effect on quality of life with ivabradine and carvedilol is most likely related to the improved exercise capacity and reduction of the beta-blocker–related fatigue.19,22
Effects on Rehospitalisation and Mortality
Although there is a short-term improvement after each admission for acute HF, patients generally leave the hospital with a further decrease in cardiac function.23 This can directly and negatively influence renal function via a decrease in cardiac output, high venous pressure or vasodilatation. After an episode of acute HF, approximately 25 % require readmission within 30 days,24 and 50 % within 6 months.25 HF hospitalisations also significantly duce the QoL of patients with HF, most of whom are older adults.
Every HF hospitalisation is a strong predictor for mortality,26 reinforcing the preventive interventions known to reduce HF hospitalisation and death. The majority of HF hospitalised patients will not see a cardiologist during the 3 months following discharge and therefore it is essential to adjust therapy, including ivabradine, before discharge.
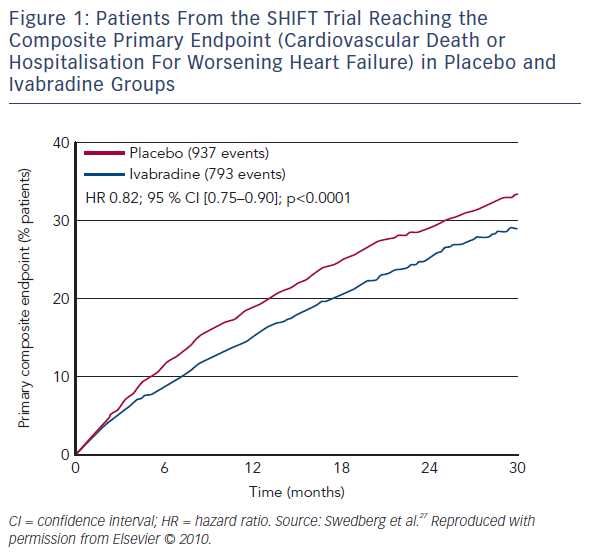
The SHIFT trial randomised 6,558 HF patients to ivabradine or placebo.27 A total of 90 % were receiving a beta-blocker, 92 % an ACEI or ARB, and 60 % MRA. In comparison with the standard clinical practice, the SHIFT patients were more adequately treated than in most countries.12,13 A reduction of 18 % in the primary composite endpoint (cardiovascular death or hospitalisation for worsening HF), as well as a 26 % reduction in hospitalisations for worsening HF and a 26 % reduction in pump-failure death were reported in the ivabradine group (see Figure 1).27 The magnitude of heart rate reduction caused by beta-blocker and ivabradine, rather than background beta-blocker dose, primarily determines subsequent effect on outcomes.28
The beneficial effects of ivabradine in SHIFT population was confirmed in all subgroups including patients with diabetes, arterial hypertension, concomitant treatment with MRAs or beta-blocker treatment and chronic obstructive pulmonary disease.29–33
Furthermore, the effect of heart rate reduction with ivabradine is maintained regardless of presence and number of co-morbidities. There was no interaction between one or more co-morbidities and the effect of treatment with ivabradine on outcomes in chronic HF.34 Age does not limit the appropriate use of ivabradine in patients with chronic HF and systolic dysfunction. The safety and efficacy of ivabradine are comparable across all age groups.35 Although heart rate is not clearly elucidated previously, the reduction in heart rate by ivabradine had no negative effect on renal function (2-year follow-up). The beneficial cardiovascular effects and safety of ivabradine were similar in patients with and without renal dysfunction.36
Safety Profile in Heart Failure
Ivabradine is well tolerated in HF patients. The prospective, openlabel multicentre PractIcal daily EffectiveNess and TolEraNce of Procoralan® in chronic SystolIc heart Failure in GermanY (INTENSIFY) study analysed the effectiveness and tolerability of ivabradine in daily practice over a 4-month period. In addition to improvements in New York Heart Association (NYHA) functional class and reduction in heart rate, the study revealed an association between ivabradine and improvement in QoL. Effects were more pronounced in patients with higher NYHA classes.37 In the SHIFT trial there was a lower incidence of serious adverse events with ivabradine than with placebo.27 In total, 21 % of patients on ivabradine discontinued treatment, versus 19 % of patients on placebo. Bradycardia was more frequent with ivabradine than with placebo (11 % versus 2 %, respectively). In addition, visual luminous phenomena (phosphenes) were reported in 3 % of patients in the ivabradine group.27 Additional safety evidence for ivabradine comes from the morBidity-mortality EvAlUaTion of the If inhibitor ivabradine in patients with coronary disease and left ventricULar dysfunction (BEAUTIFUL) trial in coronary heart disease and an LVEF less than 40 %. In this study 22.5 % patients in the ivabradine group had serious adverse events compared with 22.8 % in the placebo group. However there was a higher incidence of bradycardia (including asymptomatic bradycardia) in the ivabradine group than in the placebo group (21 % versus 2 %).38

Trimetazidine in Heart Failure
Trimetazidine offers metabolic modulation as a different treatment option in HF. Several drugs that affect cellular metabolism have been investigated over the last decades.39–41 Their mechanism of action is believed to involve inhibition of oxidation of free fatty acids in ischaemic myocytes. Since glucose metabolism requires less oxygen per mole of adenosine triphosphate generated, it is preferable to fatty acid oxidation when oxygen availability is limited in underperfused myocardium.42,43 Due to the lack of large-scale clinical trials, this clinical concept is not widely accepted and, as a result, the this drug received regulatory approval only for treatment of angina pectoris.44–47
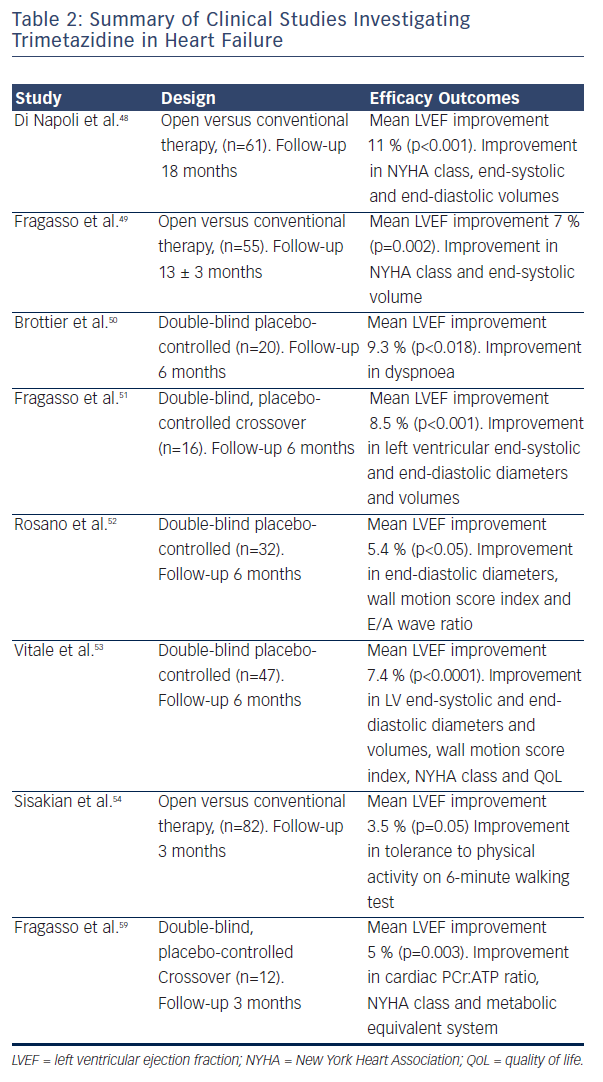
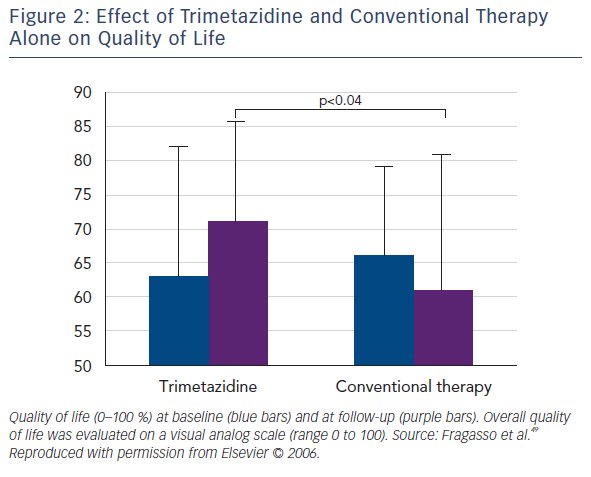
Several small randomised clinical trials (RCTs) confirmed the efficacy of trimetazidine in patients with HF. Benefical effects include improving NYHA functional class, exercise tolerance, QoL, LVEF and cardiac volumes (see Table 2).48–54 Among the first reports was the study of Brottier et al. who analysed clinical results with long-term treatment with trimetazidine on top of conventional therapy.50 After follow up of 6 months, improvement of LVEF by 9.3 % and the HF symptoms was demonstrated. In an open label study on 55 patients with HF (add-on trimetazidine versus conventional therapy), trimetazidine improved NYHA functional class significantly in comparison to the conventional therapy. Treatment with trimetazidine significantly decreased LV end-systolic volume and increased LVEF, and significantly improved QoL (see Figure 2).51 In patients with ischaemic cardiomyopathy, the trimetazidine treatment was associated not only to functional improvement and reduction in hospitalisations and mortality, but also with a significant positive effect on ventricular remodeling.48,49,55–58
In several subsequent studies, the similar finding was confirmed, based on both global and regional LV systolic function improvement, as well as the enhancement of LV diastolic function.48–50,59 Furthermore, the improvement of LV remodelling processes and the reduction of the plasma inflammatory response, natriuretic peptides, cardiac troponin levels and a recovery of the endothelium-dependent relaxation of conduit arteries was shown.48,49,56,59 These results were obtained in HF patients with ischaemic aetiology while no effects of trimetazidine in patients with HF of nonischaemic aetiology was revealed.49,60–62 Regarding co-morbidities, in pilot study of 20 patients with HF and diabetes, it was suggested that trimetazidine may be particularly beneficial in this patient group.63 The potential mechanism may include the compensation for reduced glucose uptake utilisation of myocardial cells resulting from the altered insulin levels.52
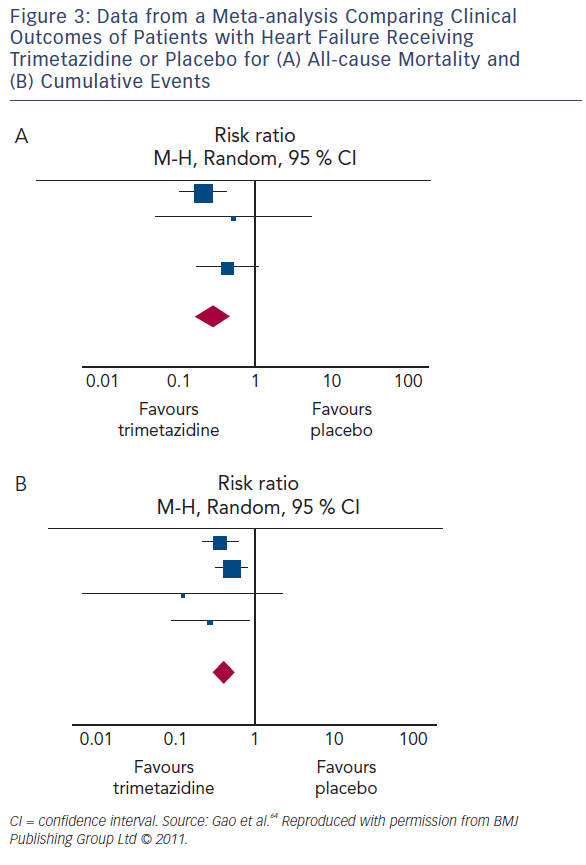
Several meta-analyses of small studies assessing the therapeutic effect of trimetazidine in HF have been published (see Table 3). Metaanalysis of 17 trials involving 955 HF patients (between 1966 and May 2010) concluded that trimetazidine therapy significantly reduces LV end-systolic volume, improves NYHA functional class and exercise duration, as well as decreasing all-cause mortality, cardiovascular events and hospitalisation (see Figure 3).64 Furthermore, metaanalysis of 16 RCTs involving 884 patients with chronic HF, highlighted that trimetazidine as add-on therapy may decrease hospitalisation for cardiac causes, improve clinical symptoms and cardiac function and simultaneously ameliorate LV remodelling.65 Another metaanalysis involving 326 patients from three RCTs reviewed the effect of adding trimetazidine to pharmacological HF therapy on all-cause mortality, concluding the beneficial effect on all-cause mortality and event-free survival.66 In a meta-analysis of 19 RCTs involving 994 HF patients on treatment with trimetazidine or placebo, trimetazidine therapy was associated with several positive effects. These include improvement in LVEF, NYHA functional class, significant decrease in LV end-systolic volume, LV end-diastolic volume, hospitalisation for cardiac causes, B-type natriuretic peptide and C-reactive protein. However, there were no significant differences in exercise duration and all-cause mortality between patients treated with trimetazidine and placebo.67
Adverse effects associated with trimetazidine have been minor and mostly gastrointestinal. However, retrospective studies have found an association between long-term use of trimetazidine and Parkinson syndrome, gait disturbances and tremor.68,69 In the majority of cases, withdrawal of the drug leads to rapid resolution of these symptoms.70 Further clinical trial data is required to determine the long-term safety of trimetazidine.71
Recommendations for the Use of Ivabradine and Trimetazidine According to the 2016 ESC Guidelines for Diagnosis and Treatment of Heart Failure
The role of ivabradine for treatment of HF was again stressed in 2016 ESC guidelines. Ivabradine is recommended in symptomatic HF patients who are in sinus rhythm with LVEF ≤35 % and heart rate higher than 70 bpm, despite treatment with optimal or maximal tolerated dose of beta-blocker. Those patients should also be on ACEI (or ARB) and MRA. This treatment was proven to reduce the risk of HF hospitalisation and cardiovascular death4 (class IIa, level of evidence B). In addition, for the patients who are unable to tolerate or have contra-indications for a beta-blocker, ivabradine is indicated with the class IIa, level of evidence C.4
Ivabradine is also recommended for the treatment of stable angina pectoris with symptomatic (NYHA Class II-IV) HFrEF, in combination with an anti-angina drug, with the exception of ranolazine and nicorandil (because of unknown safety), class IIa, level of evidence B).4 Ivabradine is not recommended in patients with atrial fibrillation in HF.4
The 2012 guidelines did not include trimetazidine in HF treatment. The 2016 guidelines indicate that trimetazidine may be considered for the treatment of stable angina pectoris with symptomatic HFrEF, when angina persists despite treatment with a beta-blocker (or alternative), to relieve angina (effective anti-anginal treatment, safe in HF), class IIb, level of evidence A.4 This recommendation is based on the body of evidence suggesting that trimetazidine may improve NYHA functional capacity, exercise duration and LV function in patients with HFrEF. There is no recommendation for trimetazidine in the setting of HF alone.
Summary and Clinical Implications
The treatment goals in patients with HF are to relieve heart failurerelated symptoms, prevent hospital admission and improve survival. Most recent therapeutic improvements are due to the use of pharmacological agents that modulate neuro-hormonal axes, ACEI, ARB, beta-blockers and MRAs. The impact of lowering heart rate on heart failure outcomes is well established and beta-blockers are recommended as first-line therapy in patients with HFrEF. Ivabradine offers further heart rate reduction and clinical and prognostic benefits in the patients on maximally tolerated dose of beta-blockers (or those intolerant to beta-blockers).
Due to its unique mechanism of action, ivabradine is now considered a well-established drug in the treatment of chronic HF. Heart rate reduction caused by ivabradine prolongs diastolic perfusion time and increases coronary blood flow and exercise capacity. The clinical effects of ivabradine in HF can be summarised as effects on rehospitalisation QoL. The clinical indications for ivabradine include all patients with symptomatic HFrEF in sinus rhythm with LVEF ≤35 % who remain with heart rate above 70 bpm, despite optimal medical therapy including maximally tolerated dose of beta-blockers. In addition, ivabradine is also recommended for the treatment of symptomatic HFrEF and stable angina pectoris, in combination with an anti-anginal drug for patients intolerant to beta-blockers. Ivabradine has a good tolerability and safety profile.
Trimetazidine acts directly at cardiac cell level by inhibition of oxidation of free fatty acids in ischaemic myocardium, offering metabolic modulation, as a different treatment option. Potential beneficial effects are the improvement of NYHA functional class, exercise tolerance, QoL, LVEF and cardiac volumes. In clinical practice, trimetazidine may be considered for the treatment of stable angina pectoris with symptomatic HFrEF, when angina persists despite treatment with a beta-blocker (or alternative), to relieve angina. Adverse effects associated with trimetazidine have been minor and mostly gastrointestinal, although the association between the long-term treatment with trimetazidine and Parkinson’s disease, gait disturbances and tremor need to be further investigated.