Since the 1990s, the publication of several large randomised controlled trials (RCTs) have established the efficacy of implantable cardioverter defibrillator (ICD) therapy in reducing the risk of sudden cardiac death (SCD) in high-risk patients. Collectively these trials have demonstrated a significant all-cause mortality reduction compared with medical therapy alone, for both the primary and secondary prevention of SCD.1–9
The main life-saving therapy delivered by ICDs is shock therapy. In the largest cohort of real-world ICD recipients, totalling nearly 200,000 patients, 1-year shock occurrence post-implantation was 14 % and at 5 years 38 %.10 Rates of therapy are considerably higher in secondary prevention populations with nearly half receiving shocks at 1 year.11 However, although often life-saving, ICD shocks have a number of negative effects, including psychological morbidity and reduced quality of life, and are an economic burden. Data from both RCTs and observational studies have demonstrated significant reductions in measures of physical and mental wellbeing after a single shock that further decline with increasing numbers of shocks.12–17 ICD shocks also result in increased healthcare utilisation and a reduction in device longevity.
Furthermore, more worryingly sub-analyses of the major ICD trials have suggested a subsequent increased risk of death in patients that receive shocks. Whether ICD shocks are merely markers of, or are directly contributing to, the poor prognosis observed in patients receiving them is unclear. The aim of this article is to review the current literature regarding this issue.
Relationship Between Shocks and Mortality
The publication of the landmark primary prevention ICD trials, the Multicenter Automatic Defibrillator Implantation Trial (MADIT-II) and the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT), over a decade ago, resulted in a widespread increase in ICD implantation worldwide.3,6 However, subsequent re-analysis of these trials raised concerns over an adverse prognosis in patients that received ICD shocks.
The MADIT-II established the benefit of ICD implantation in postmyocardial infarction (MI) patients with a left ventricular ejection fraction (LVEF) ≤30 %, who had no history of sustained ventricular arrhythmias. However, there were initial concerns over a trend towards excess heart failure admissions in the ICD arm.3 In a subsequent analysis, the long-term follow-up data of the 720 defibrillator recipients in the trial were examined. Over a period of 21 months, 23 % of patients received device therapies for ventricular tachycardia (VT)/ventricular fibrillation (VF), which was associated with a 1-year mortality rate of 20 %. Furthermore, admissions for heart failure occurred in 26–31 % following device therapy versus 19 % in the therapy-free cohort. Hazard regression analysis demonstrated a threefold increase in mortality after a first therapy for VT (hazard ratio [HR] 3.4, 95 % confidence interval [CI] 1.9–5.9, p<0.001) or VF (HR 3.3, 95 % CI 1.3–8.1, p=0.01).18 In a further analysis of the MADIT II, inappropriate shocks were delivered in 83 (12 %) patients and associated with a twofold increase in mortality.19
In the SCD-HeFT, 2,521 patients with heart failure of any aetiology and LVEF ≤35 % were randomised to placebo, amiodarone or an ICD. Defibrillator therapy was associated with a significant 23 % reduction in all-cause mortality compared with placebo.6 Poole et al. examined the significance of shocks, both appropriate and inappropriate, in the 811 ICD recipients. During a follow-up of 46 months 33 % received at least one shock, of which only 47.6 % were solely for VT/VF, 32.3 % for non-VT/VF and 20.1 % for both. In multivariate analysis, both inappropriate shocks (HR 1.98, 95 % CI 1.29–3.05, p=0.002) and appropriate shocks (HR 5.68, 95 % CI 3.97–8.12, p<0.001) were associated with mortality. The risk further increased with additional appropriate shocks, which was associated with an eightfold risk of death, and the occurrence of inappropriate shocks on top of this, increased this further to a nearly 16-fold risk.20
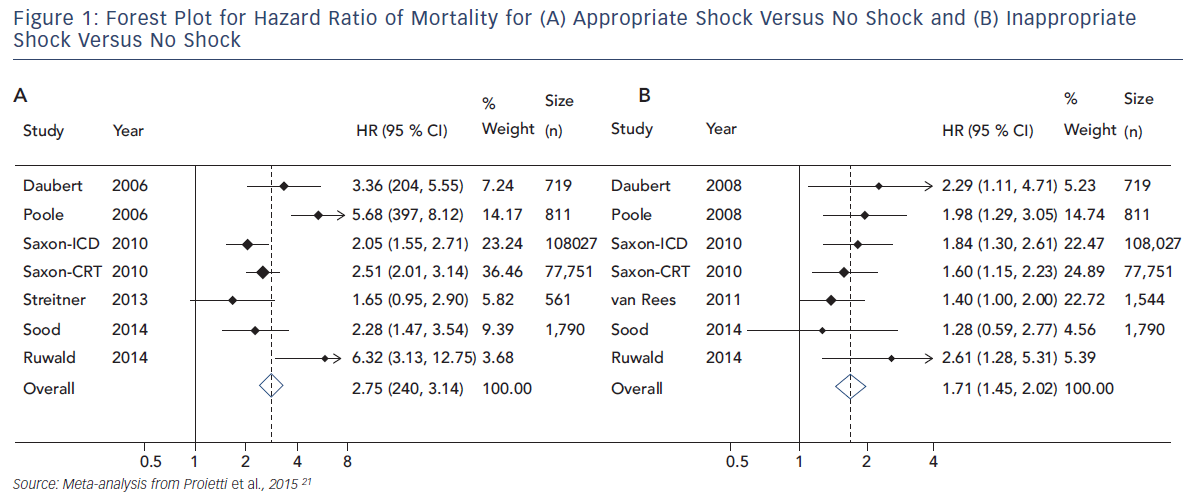
Proietti et al. performed a meta-analysis examining the size of the association between ICD shocks and mortality in major ICD trials. Data from 10 studies, including nearly 200,000 patients, were evaluated. In a pooled analysis, a significant association was found between ICD shocks and mortality. The association was stronger for appropriate (HR 2.95, 95 % CI 2.12–4.11, p<0.001) than inappropriate shocks (HR 1.71, 95 % CI 1.45–2.02, p<0.001) but both associations were significant. In keeping with prior studies, the combination of appropriate and inappropriate shocks was associated with a greater risk than the occurrence of just one type of shock (see Figure 1).21
These data conclusively demonstrate that the occurrence of ICD shocks is associated with a worse prognosis. The risk appears to be greater for appropriate than inappropriate shocks, though both are associated with impaired survival. Furthermore, multiple shocks are associated with worse outcomes.
Potential Mechanism of Increased Mortality with Shocks
Evidence from human studies has shown that shocks increase serum biomarker levels of myocardial injury;22–24 however, translation to increased mortality has not been established.
Experimental studies have also suggested that shock delivery may lead to direct myocardial stunning, the degree of which is related to the magnitude of the electrical shock. This is a mechanism that may be responsible for the post-shock pulseless electrical activity (PEA) seen frequently in the post-resuscitation setting.25,26 One study reviewed 320 deaths in ICD recipients enrolled in device trials to attempt to define the mode of death. Deaths were classified using data from medical notes, interviews of witnesses and ICD memory logs. In total, 317 deaths had sufficient data to assign a mode of death; 28 % were classified as sudden, 49 % non-sudden and 22 % non-cardiac. Of the sudden deaths, post-shock PEA was classified as the mechanism in 29 %.26
This potential importance of myocardial stunning is supported by data from Toh et al., who investigated the acute effects of ICD shocks on haemodynamics and myocardial function. Fifty patients undergoing ICD implantation and defibrillation testing (DFT) were evaluated by echocardiography, serum biomarker measurements before, immediately following and at 5 minutes and 4 hours after shock delivery, and had invasive arterial pressure monitoring during the procedure. Compared with patients with LVEF >45 %, those with poorer function experienced further transient depression of LVEF until 5 minutes post-DFT, which recovered to baseline by 4 hours, and significantly longer recovery time of mean arterial pressure to baseline.27
Examining the timing of death in relation to ICD shocks in RCTs may provide further insight. Of the 811 patients in the SCD-Heft who had an ICD implanted, 269 received at least one ICD shock. Of these 269 ICD recipients 77 died; progressive heart failure accounted for 43 % of deaths and sudden arrhythmic death 21 %. Median time to death following any shock was 204 days (interquartile range 1–630) and was longer for inappropriate compared with appropriate shocks. Postmortem data were available for 64 of the 173 patients that died with the device in situ. Of these, 20 were found to have died within 24 hours of a shock.20 While myocardial stunning may potentially account for death within the first 24 hours of a shock, it is unlikely to be an important factor in patients that died sometime after their device therapy.
Is It Shocks or Progression of the Substrate?
As detailed above, there are a number of potential mechanisms by which shocks may directly increase the risk of death. However, equally there is a clear rationale for suggesting that shocks may be a marker of a higher risk patient. The occurrence of VT or VF that leads to appropriate shocks is increasingly likely in the presence of more advanced myocardial disease, which itself portends an adverse prognosis. Furthermore, the presence of atrial fibrillation (AF), the commonest cause of inappropriate therapy, is independently associated with an increased risk of death in patients with heart failure.28
Therefore, the association between ICD shocks and increased mortality may be explained by either the detrimental effects of the shocks themselves, progression of the underlying disease process (with shocks merely a marker of disease progression) or a combination of the two.
Several lines of evidence may be useful to disentangle this association. These include data from the occurrence of shocks in the absence of spontaneous arrhythmias, the effect of non-shock therapies (i.e. antitachycardia pacing [ATP]) in the treatment of ventricular arrhythmias and the impact of a range of strategies used to reduce the burden of shock therapy (strategic ICD programming, antiarrhythmic drugs and VT ablation).
The Impact of Shocks Without Spontaneous Arrhythmia
There are a number of situations in which ICD shocks are delivered without the occurrence of spontaneous arrhythmias. These include defibrillation testing at implant, the induction of VF remote from the initial implant procedure and the occurrence of shocks for nonarrhythmic causes.
Defibrillation testing in patients receiving ICDs provides an opportunity to examine the effect of shocks without acute arrhythmia. The Shockless Implant Evaluation (SIMPLE) trial was a randomised single-blind non-inferiority trial of defibrillation testing versus no defibrillation testing at the time of ICD implantation in 2,500 patients. A total of 1,253 patients were randomised to the defibrillation testing arm, of whom 74 % had primary prevention devices and 64 % an underlying ischaemic cardiomyopathy. The mean LVEF was 32 %. Overall, there was no difference in the composite primary endpoint of failed appropriate shock or arrhythmic death, and no difference in all-cause mortality between the groups. Analysis of secondary endpoints showed a non-significant trend towards an increase in adverse outcomes in the defibrillation testing group (4.5 % versus 3.2 %, p=0.08). Furthermore, in the defibrillation group there was a greater need for chest compressions (0.4 % versus 0.0 %, p=0.06) and emergency intubation (0.6 % versus 0.1 %, p=0.03) compared with the control arm.29 These data possibly suggest some detrimental effect of receiving shocks without spontaneous arrhythmia, though any potential effect is small and of uncertain clinical significance.
Data on the prognostic implication of induced VF is provided by Bhavnani and colleagues who followed up a cohort of 1,327 patients undergoing ICD implantation from a single centre. All patients underwent ICD implantation with defibrillation testing after VF induction during the procedure. Patients were stratified into four groups according to shock type received: implantation shocks only, additional shocks for non-invasively stimulated VF, additional appropriate shocks only and additional inappropriate shocks only. A combined primary endpoint of all-cause mortality and hospitalisation for acute decompensated heart failure was used. When compared with implantation-only shocks, patients who underwent non-invasive VF induction with subsequent shocks had a similar risk of death and hospitalisation for heart failure. However, the occurrence of spontaneous arrhythmias requiring shocks carried a twofold risk of death and heart failure hospitalisation.30
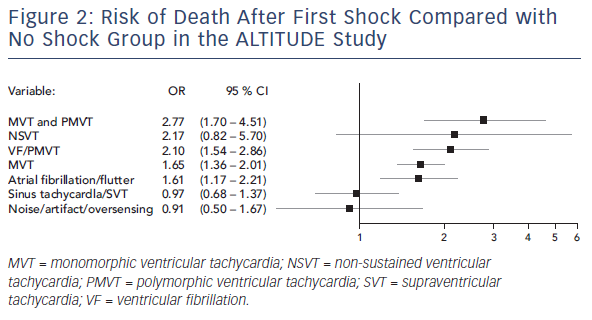
The ALTITUDE study aimed to differentiate the risk associated with shocks versus the underlying rhythm in patients receiving only inappropriate shocks. This was a prospective observational study of 127,134 patients who had either an ICD or cardiac resynchronisation therapy defibrillator (CRT-D) and were followed using a remote monitoring system. From this cohort, the investigators randomly sampled 3,809 (13 %) patients who received ≥1 shock. Over a 3-year follow up, 41 % of patients received shocks for non-VT/VF rhythms. Atrial arrhythmias were the commonest cause, accounting for 44 %, followed by other supraventricular arrhythmias (41 %) and noise or oversensing (11 %). In matched comparison to the no-shock group, the risk of death was no different if an inappropriate shock was delivered due to supraventricular arrhythmias (HR 0.97, 95 % CI 0.68–1.37, p=0.86) or noise/oversensing (HR 0.91, 95 % CI 0.50–1.67, p=0.76). In contrast, shocks delivered for AF/atrial flutter were associated with an increased risk of death (HR 1.61, 95 % CI 1.17–2.21, p=0.003) (see Figure 2).31 These findings were also replicated by a smaller prospective study of 1,411 patients.32
These data, evaluating the impact of shocks occurring in the absence of spontaneous arrhythmias, suggest that the risks associated with shocks are predominantly due to the underlying rhythm rather than the shocks themselves.
The Relationship Between Antitachycardia Pacing and Mortality
ATP was developed as an alternative therapy to terminate VT to avoid shocks. Examining the association of mortality with ATP therapy may provide contributory evidence in the substrate versus shocks debate. However, data from individual studies are conflicting with some showing an increased risk of death and others not.
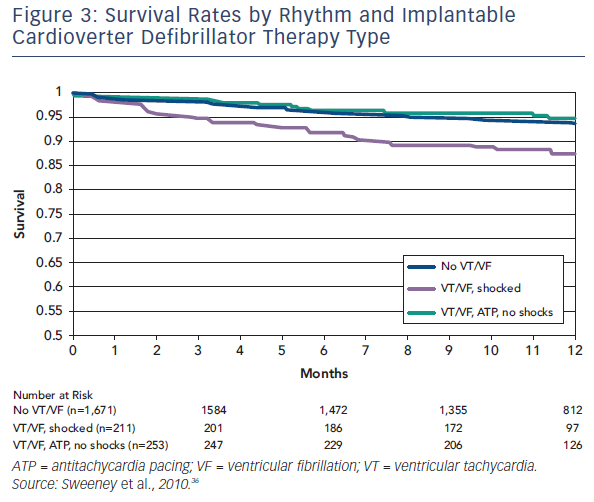
Sweeney et al. published data from a meta-analysis of 2,135 patients enrolled in four trials that used ATP to reduce ICD shocks.33 Patients were predominantly male with ischaemic heart disease, and the majority received prophylactic devices. Over 11 months of follow-up, 24.3 % patients received appropriate device therapy and 6.6 % died. Analysis of the differential effect of shocks versus ATP could only be ascertained for fast VTs, defined as 188–250 bpm, as slower VT were predominantly treated with ATP and VF with shocks. Shocks for fast VT were associated with an increased risk of death (HR 1.32, 95 % CI 1.23–1.41, p<0.0001), whereas ATP had no effect (see Figure 3).
In a more recent study, Kleeman et al. prospectively followed 1,398 patients who underwent ICD implantation in a single centre. Patients were stratified into groups according to the mode of therapy: ATP only termination, appropriate shock termination or no appropriate therapy of any type. Over a 6-year follow-up, 54 % required therapies to terminate VT/VF. Of these, 74 % were terminated by ATP only. In multivariate analysis, an episode of first ATP was associated with a 2.6-fold increased risk of death (95 % CI 2.02–3.35). This association remained significant when excluding patients with appropriate shocks after prior ATP (HR 1.92, 95 % CI 1.38–2.67). However, the risk associated with ATP was still lower than that seen with shock therapy.34 A similar association with ATP-only therapy compared with no therapy was also demonstrated in the recently reported Assessing Therapies in Medtronic Pacemaker, Defibrillator and Cardiac Resynchronization Therapy Devices (OMNI) trial involving 2,255 patients over a 3-year follow-up (HR for death 1.45, 95 % CI 1.05–2.02, p=0.025).35
Overall, data regarding ATP and mortality risk are conflicting. Although analysis of RCTs with relatively short follow-up have found no association between ATP and mortality, evidence from cohorts with longer follow-up appear to indicate that ATP is associated with increased mortality, though with a lesser magnitude than the association with appropriate shocks. This may suggest that the arrhythmia itself has more of a bearing on mortality, though therapy type may also have an additional contribution to risk.
Interpretation of the relationship between ATP, shocks and mortality is further complicated by the fact that ventricular arrhythmias treated with ATP are typically different from those treated with shocks.18 Ventricular rates tend to be lower, arrhythmia onset to therapy delivery is typically shorter due to the absence of a charge time with ATP, and overall VT amenable to ATP may be a marker of a less diseased myocardium. Early studies of ATP testing for induced VT demonstrated lower success rates and higher rates of tachycardia acceleration for faster VTs.36 Furthermore, data reported by Moss and colleagues suggested that slower VTs, which are more frequently terminated by ATP, were associated with a better outcome than that of fast VTs.18 In another study, the rate of ICD shocks preceded by failed ATP was 18 times higher in patients who died at follow-up, further supporting the hypothesis that VT unresponsive to ATP could be a marker of substrate severity.33
Strategies to Reduce Implantable Cardioverter Defibrillator Shocks and Their Effect on Mortality
Strategies that have been shown to reduce the burden of ICD shocks are summarised in Table 1, and their clinical impact on morbidity and mortality are discussed below.
Impact of Implantable Cardioverter Defibrillator Programming on Shock Reduction
Strategic ICD programming can reduce the occurrence of ICD therapy without altering the underlying myocardial substrate, and has provided clear evidence implicating shocks as directly influencing mortality risk.37 Two recent meta-analyses have examined the effect of ICD programming strategies on mortality reduction.
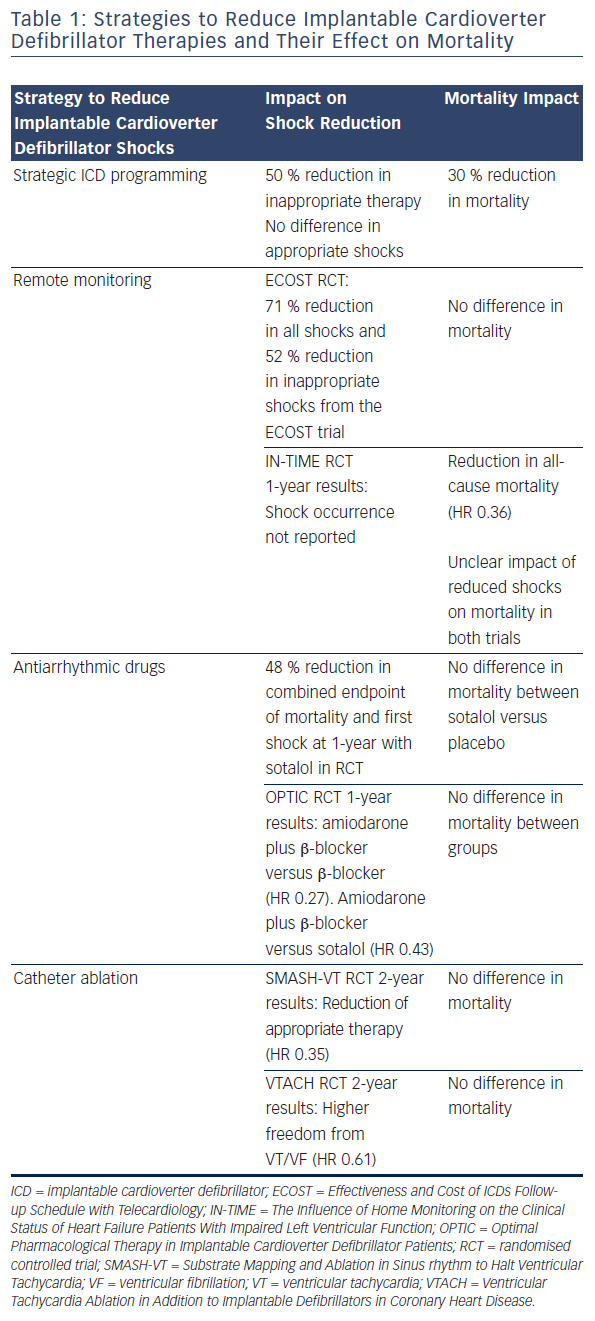
Tan et al. sought to quantify the overall effect of ICD therapy reduction programming strategies on mortality from six major programming trials: Comparison of Empiric to Physician-tailored Programming of Implantable Cardioverter Defibrillators (EMPIRIC), Primary Prevention Parameters Evaluation (PREPARE), Role of Long Detection Window Programming in Patients With Left Ventricular Dysfunction, Non-ischemic Etiology in Primary Prevention Treated with a Biventricular ICD (RELEVANT), Multicenter Automatic Defibrillator Implantation Trial-Reduce Inappropriate Therapy (MADIT-RIT), Avoid Delivering Therapies for Nonsustained Arrhythmias in ICD Patients III (ADVANCE III) and Programming Implantable Cardioverter- Defibrillators in Patients with Primary Prevention Indication to Prolong Time to First Shock (PROVIDE). In total 4,089 patients with therapy reduction programming were compared with 3,598 conventionally programmed patients. Therapy reduction programming involved using combinations of long detection times, high detection rates and SVT discriminators. Over a 1-year follow-up there was a 50 % reduction in inappropriate shocks in the strategic programming group, though appropriate shock rates were similar between groups. Therapy reduction programming was associated with a 30 % reduction in mortality (95 % CI 16–41 %, p<0.001) compared with the conventional arm (see Figure 4).38
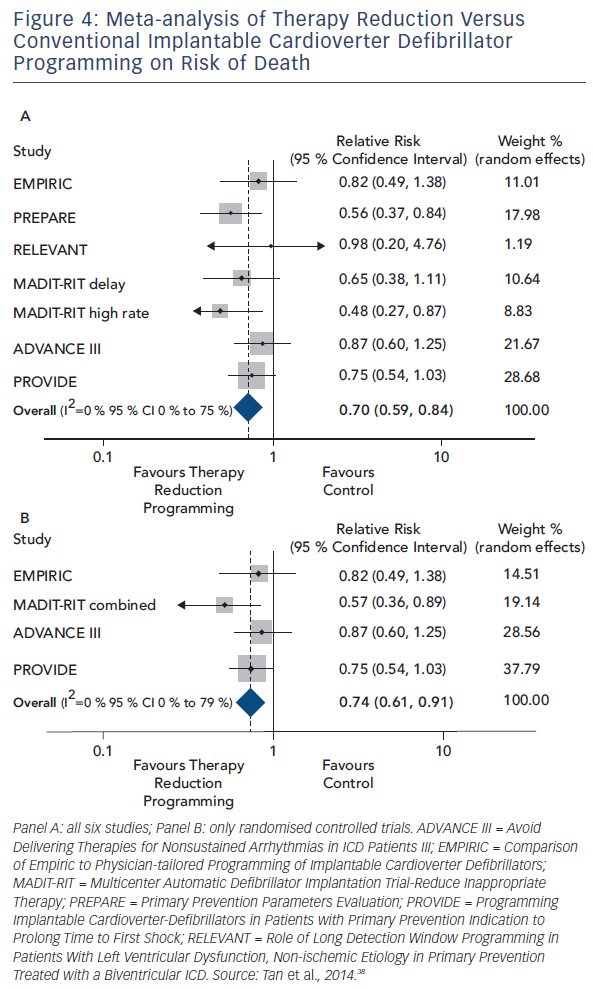
The mortality benefit of programming long detection times was the focus of a meta-analysis by Scott and colleagues. Four studies enrolling 4,896 patients were included: RELEVANT, MADIT-RIT, ADVANCE III and PROVIDE. A mortality reduction of 23 % (RR 0.77, 95 % CI 0.62–0.96, p=0.02) was seen in the long detection arm. In keeping with the analysis of Tan et al. there was a 50 % reduction in inappropriate shocks, but no significant difference in the occurrence of appropriate shocks. Importantly, no increase in risk of syncope was seen. Data on ATP therapy was derived from two studies, which indicated a substantial reduction in both appropriate (RR 0.25, 95 % CI 0.15–0.41) and inappropriate ATP (RR 0.35, 95 % CI 0.19 – 0.64).39
The mortality reduction seen with strategic programming is compelling evidence that shock therapy and possibly ATP as well are not only markers of risk but have a direct and significant impact on mortality.
Remote Monitoring of Implantable Cardioverter Defibrillators
Modern ICDs now have the ability to be remotely monitored. Data can be automatically transmitted from the device following a detected event or change in certain physiological parameters, which is then sent to a central database and onto the local device clinic, usually within 24 hours.40 Remote monitoring (RM) enables early detection of clinical or device-related problems and allows prompt intervention. Other potential benefits include reducing unnecessary face-to-face visits where a patient’s clinical status has remained stable, thus reducing the economic burden.41
The role of RM in reducing both appropriate and inappropriate shocks has been the subject of several studies. In a sub-analysis of the Effectiveness and Cost of ICDs Follow-up Schedule with Telecardiology (ECOST) trial involving 433 randomised patients, RM significantly reduced the number of shocks of any cause by 71 %, driven mainly by a 52 % reduction in inappropriate shocks and with a subsequent 72 % reduction in hospitalisation.42 Sensing problems arising as a result of lead failure, electromagnetic interference, T wave or myopotential oversensing contribute a small but important proportion of inappropriate shocks and can be preceded by detected events prior to any therapy administered. In one small single-centre cohort study of leads under advisory, RM patients experienced reduced shocks compared with those with standard clinic follow-ups (27 % versus 47 %).43
The ALTITUDE study, which compared nearly 70,000 patients under RM to 116,000 patients with device clinic only follow-up, provided compelling mortality data. The main finding of this study was a striking 50 % relative reduction in the risk of death in patients with RM (HR 0.56 for ICD, 0.45 for CRT-D). However, clinical data were lacking and as such differences in baseline characteristics that could have influenced survival could not be adjusted for between the groups.10 In the Influence of Home Monitoring on the Clinical Status of Heart Failure Patients With Impaired Left Ventricular Function (IN-TIME) multicentre RCT of automatic daily RM versus standard care in 664 patients, 1-year all-cause mortality was lower in the RM monitoring group (HR 0.36, p=0.004).44 Although mortality reduction was seen in both these trials, it is not possible to determine the contribution of shock reduction on overall mortality with these data.
The Impact of Antiarrhythmic Therapy on Shock Reduction and Mortality
Therapy with antiarrhythmic drugs (AADs) aims to reduce the burden of arrhythmias associated with both appropriate and inappropriate therapy. As such AAD therapy may reduce device therapy by altering the electrophysiological properties of the myocardial substrate without significantly altering the underlying myocardial architecture.
Two randomised trials have systematically examined the effect of AAD therapy in reducing ICD shocks. Sotalol was compared with a placebo in a double blind trial of 302 secondary prevention ICD patients. A combined primary endpoint of all-cause mortality and first shock therapy for any cause was used. At 1-year follow-up, sotalol was associated with a 48 % reduction in the primary endpoint compared with placebo, with a greater reduction in inappropriate versus appropriate shocks.45 There was no difference in mortality between the sotalol (four deaths) and placebo groups (seven deaths). The Optimal Pharmacological Therapy in Implantable Cardioverter Defibrillator Patients (OPTIC) trial randomised 412 patients with recently implanted ICDs, clinically documented VT/VF and a LVEF ≤40 % to a combination of amiodarone plus β-blocker, sotalol or β-blocker alone. At 1-year follow-up patients in the amiodarone plus β-blocker arm experienced fewer shocks compared with either sotalol (HR 0.43, 95 % CI 0.22–0.85) or β-blocker alone (HR 0.27 CI 0.14–0.52). There was a non-significant trend towards fewer shocks with sotalol compared with β-blockers alone (HR 0.61, 95 % CI 0.37–1.01, p=0.055). Rates of AAD discontinuation were higher with amiodarone (18 %) and sotalol (24 %) compared with β-blockers alone (5.3 %). Amiodarone was also associated with a high number of pulmonary (5.0 %) and thyroid (5.7 %) complications. Overall the mortality rate was low (3.1 % at 1-year) with no difference between treatment groups.46
The two studies taken together suggest that AADs significantly reduce the frequency of ICD shocks, without a significant mortality benefit. However the studies were not powered to demonstrate any mortality benefit. Furthermore, it is possible that any potential prognostic benefit from shock reduction may be offset by drug-related adverse events.47
Ventricular Tachycardia Ablation to Reduce Implantable Cardioverter Defibrillator Shocks
Ablation has become increasingly important as an adjunctive therapy to reduce shocks in ICD recipients. There have been two RCTs of prophylactic VT ablation in patients with ICDs implanted after documented VT/VF due to ischaemic heart disease.
The Substrate Mapping and Ablation in Sinus rhythm to Halt Ventricular Tachycardia (SMASH-VT) trial was a prospective, randomised, multicentre trial of catheter ablation versus medical therapy alone in 128 post-MI patients with a recently implanted secondary prevention ICD. The primary endpoint was freedom from any ICD therapy, either ATP or shocks. After a 2-year follow-up, catheter ablation reduced any appropriate therapy from 33 % to 12 % (HR 0.35, 95 % CI 0.15– 0.78, p=0.007) and appropriate shocks from 31 % to 9 % (p=0.003); however, mortality did not differ between the groups.48
The Ventricular Tachycardia Ablation in Addition to Implantable Defibrillators in Coronary Heart Disease (VTACH) trial was of a similar design to the SMASH-VT trial and enrolled 107 patients to catheter ablation plus ICD or ICD alone. In contrast to the SMASH-VT trial patients, those enrolled in the VTACH trial were a more homogenous group that required documented stable VT after baseline VT induction and had an ICD implanted post-ablation. At 2-year follow-up, time to VT/VF recurrence was longer in the ablation arm, 18.6 versus 5.9 months, and freedom from VT/VF was higher, 46 % versus 29 % (HR 0.61 95 % CI 0.37–0.99). Again, mortality did not differ between the two arms.49
To capture any possible effect of VT ablation on mortality, Mallidi et al. analysed data on 457 patients from five studies, including the SMASH-VT and VTACH populations, along with three observational studies. Catheter ablation was associated with a 35 % reduction in VT recurrence but with no effect on mortality. However, significant procedural complications occurred in 6 %, including death, stroke/transient ischaemic attack (TIA), cardiac perforation and atrioventricular (AV) block (approximately 1 % each), which may have offset any potential benefit on mortality seen due to a reduction in shocks.50
The results of ongoing VT ablation trials such as the Does Timing of VT Ablation Affect Prognosis in Patients With an Implantable Cardioverter-defibrillator? (PARTITA), Ventricular Tachycardia Ablation or Escalated Drug Therapy (VANISH)51 and Preventive Ablation of Ventricular Tachycardia in Patients with Myocardial Infarction (BERLIN) should provide further clarification on the relationship between reduction in ICD shocks and mortality.
The Relationship Between Left Ventricular Remodelling and Shocks
Sood et al. examined the relationship between the degree of post-implant left ventricular remodelling, the occurrence of ICD shocks and mortality. The study population comprised 1,790 patients who received either an ICD or CRT-D as part of the MADIT-CRT study. Myocardial substrate progression was assessed by standard transthoracic echocardiography at baseline and at 1-year follow-up using LVEF and indexed LV volumes. Advanced myocardial structural disease, i.e. higher baseline echocardiographic volumes and lack of left ventricular remodelling at 1-year, was present in patients who received appropriate shocks but not in patients who received inappropriate shocks or no shocks. At 2-year follow-up, patients that received appropriate (HR 2.3, 95 % CI 1.47–3.54, p<0.001) but not inappropriate shocks (p=0.42) had an increased risk of mortality. This association remained significant when adjusted for echocardiographic remodelling at 1 year.
This study suggests that the occurrence of shocks and the presence of advanced myocardial substrate remodelling are inextricably linked, and that the deleterious effects of shocks are most marked in the presence of a more diseased myocardial substrate.52
Conclusion
It is clear that there is a strong and consistent association between increased mortality and both inappropriate and appropriate shocks. However, disentangling whether shocks are purely a marker of the severity of the underlying cardiac disease or whether they directly contribute to risk is challenging.
Data supporting shocks as only a marker of risk include the neutral effect of shocks occurring in the absence of spontaneous arrhythmias, such as in defibrillation testing and inappropriate shocks for a nonarrhythmic cause. In contrast, data from trials examining the role of ICD programming to reduce shocks provide compelling evidence that shocks themselves contribute to risk.
Overall the data are inconclusive. However, although it is not possible to draw definitive conclusions it is likely that both the substrate and the occurrence of shocks are important. It may be that while the occurrence of ICD shocks is a marker of more advanced cardiac disease, which itself portends a poor prognosis, the occurrence of shocks in the presence of a diseased substrate adds additional incremental risk that can be reduced by the avoidance of unnecessary shocks. This hypothesis is supported by data from the MADIT-CRT study examining the relationship between mortality, shocks and substrate progression.
However, what is incontrovertible is that ICD shocks are physically unpleasant and psychologically damaging, and so reducing them is important irrespective of their prognostic significance. Furthermore, the most common mode of death in ICD patients receiving a shock is pump failure, and the occurrence of any ICD therapy should prompt the re-evaluation and aggressive treatment of heart failure.
Clinical Perspective
- ICD shocks are associated with increased mortality.
- It is unclear whether shocks are merely a marker of a more severe disease or directly contribute to mortality.
- Shocks in the absence of spontaneous arrhythmia have a neutral effect on mortality.
- Reducing shocks by ICD programming reduces mortality.
- Regardless of prognostic implication of shocks, they are painful, psychologically detrimental and should be avoided.