The diagnosis and treatment strategies for coronary artery disease are traditionally based on the percentage of coronary angiographic vessel stenosis. We are witnessing a gradual transition from angiographic evaluation of individual coronary artery lesions towards the combination of anatomy and physiology to determining its physiological consequences. With the introduction and rapid evolution of fractional flow reserve (FFR) technology, a new gold standard has been developed to invasively assess the physiological severity of a coronary artery stenosis.1–3 Fractional flow reserve provides a real-time measurement of the extent to which a given epicardial stenosis limits maximal myocardial flow and identifies lesions that should be corrected by revascularisation.
The Impact of FFR Technology on Revascularisation Strategies
Various trials helped to support the premise that percutaneous coronary interventions (PCI) should be guided more by physiological considerations and not solely by anatomic factors.4,5,7
A number of studies designed to determine the role of FFR on coronary artery bypass grafting (CABG) have been done with promising results; however, larger prospective randomised trials are needed.10 Additionally, we still do not know what the long-term effects of not grafting angiographic stenotic lesions will be on the distal myocardium.11–13
Clinical Examples that Illustrate the Disparity Between Angiographic and FFR Lesion Evaluation
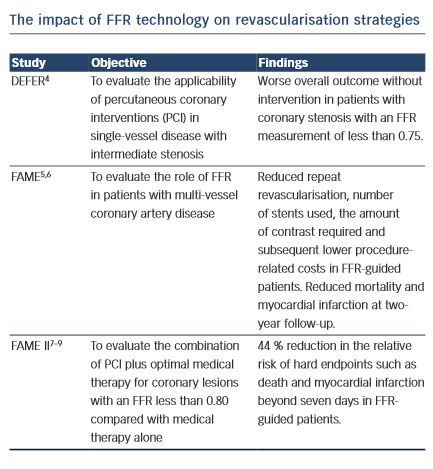
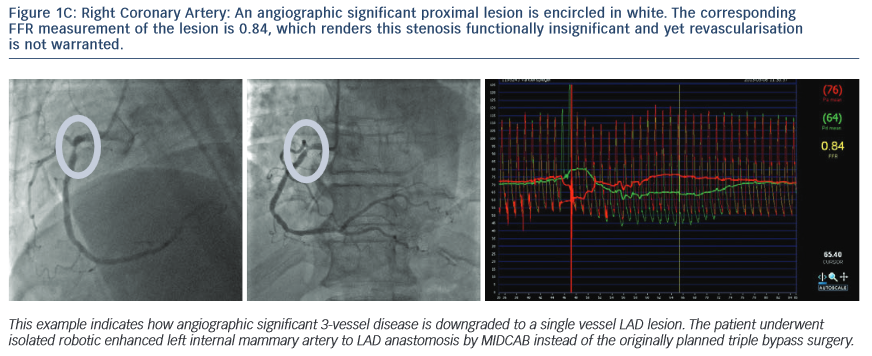
1.The Following Images Describe Significant Angiographic 3-Vessel Disease in a Patient with Stable Angina
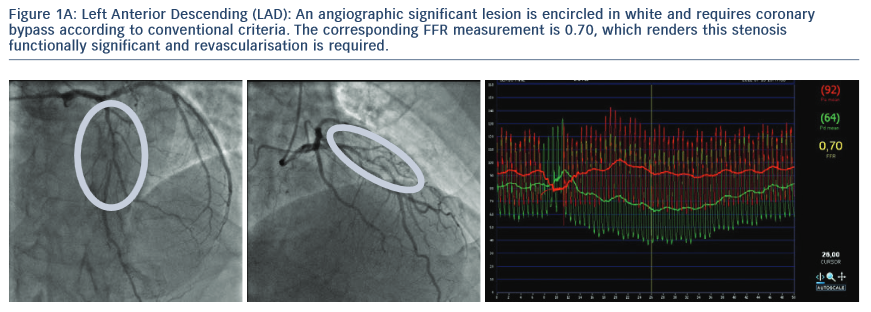
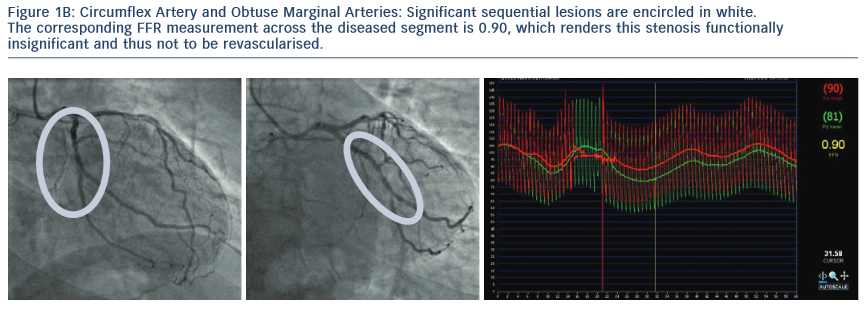
2. The Following Images Describe the Angiograms of Lesions Judged to be Potentially Insignificant in Two Separate Patients with Stable Angina
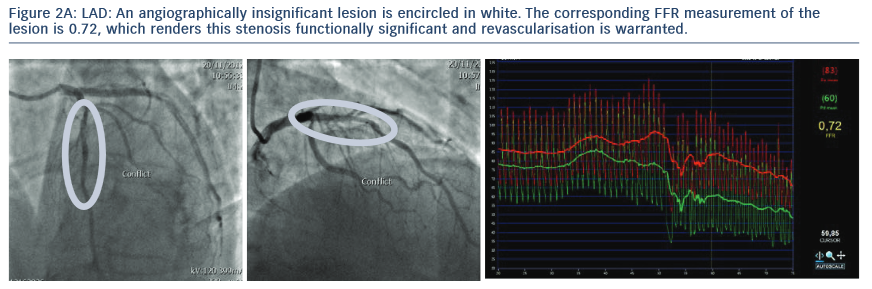
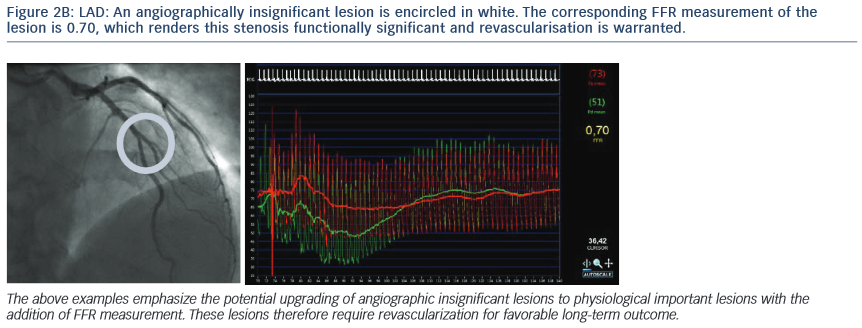
Conclusion
FFR technology has proven its value in decision making about percutaneous coronary interventions but before changes are made in determining what vessels should and should not be grafted during CABG, larger prospective randomised trials with longer follow-up are needed to better understand the role of this technology in CABG. FFR-guided CABG is now under intense investigation and may have an important role in determining whether angiographic lesions should be bypassed.
Rx Only
Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.
Unless otherwise noted, TM indicates that the name is a trademark of, or licensed to, St. Jude Medical or one of its subsidiaries. ST. JUDE MEDICAL and the nine-squares symbol are trademarks and service marks of St. Jude Medical, Inc. and its related companies. Article courtesy of Filip Casselman MD, PhD, FETCS, Johan Van der Merwe MD, MMED (Thorax).